��Ŀ����
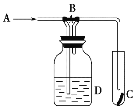
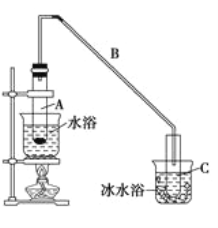
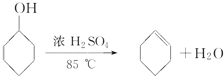
����Ŀ��ij��ѧС�������ͼ��ʾ��װ�ã��û������Ʊ�����ϩ��
��֪��
��Է������� | �ܶ�/g cm-3 | �۵�/�� | �е�/�� | �ܽ��� | |
������ | 100 | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 82 | 0.81 | -103 | 83 | ������ˮ |
��1���Ʊ���Ʒ
��12.5 mL��������1mLŨ��������Թ�A�У�ҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
�����Թ��л�ϻ��Ѵ���Ũ�������ʱ������ҩƷ���Ⱥ�˳��Ϊ______________________��
���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ������_________������ĸ����
A.�������� B.��ȴ�� C.���貹�� D.��������
�۽��Թ�C���ڱ�ˮ�е�Ŀ����_______________________________________________��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����Ʒ�м��뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_______��(����������������)����Һ����________������ĸ��ϴ����
a.����KMnO4��Һ b.ϡ���� c.Na2CO3��Һ
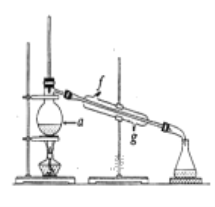
���ٽ��ᴿ��Ļ���ϩ����ͼ��ʾװ�ý�������ͼ������a ��������_______________,ʵ������ȴˮ��______������ĸ���ڽ���������ʱҪ������ʯ�ң�Ŀ����__________________________��
��3�������Ʊ���Ʒʱ���Ѵ����Ʒһ����������ʵ���ƵõĻ���ϩ��Ʒ����_______(�������������� ������)���۲�������ʵ�����õ��Ļ���ϩ����Ϊ6.5g���������______������һλС������
���𰸡� �Ƚ������������Թ�A�У��ٻ�������Ũ���� B ��ֹ����ϩ�Ļӷ�������������Ҳ������ �� c ������ƿ g ����ʣ���ˮ ���� 66.1%
����������1����Ũ����ϡ�Ͳ����������ȣ��������Թ��л�ϻ��Ѵ���Ũ�������ʱ������ҩƷ���Ⱥ�˳��Ϊ�Ƚ������������Թ�A�У��ٻ�������Ũ����ʴ�Ϊ���Ƚ������������Թ�A�У��ٻ�������Ũ���
�����ƬΪ��ֹҺ�屩�����ã�����һ��ʱ��������Ǽ����ƬӦ��ȴ�ӣ���ѡB���ʴ�Ϊ��B��
�۱�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ������ֹ����ϩ�Ļӷ����ʴ�Ϊ����һ����ȴ����ֹ����ϩ�ӷ���
��2���ٻ���ϩ�����࣬�������Ȼ�����Һ�����ܶȱ�ˮС�������á��ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ����������룺�Ʊ����������ᴿ����ʱ��c��Na2CO3��Һ��ϴ�ӿɳ�ȥ�����ʴ�Ϊ���ϣ�c��
������������״��֪��aΪ������ƿ��Ϊ����������Ч��������װ��Ҫ�������ܣ���ȴˮ���¿ڣ�g�����룬����ʱҪ������ʯ�ҿ�������ʣ���ˮ���ʴ�Ϊ��������ƿ��g������ʣ���ˮ��
��3���ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�����������������Ϊ��0.96g/mL��12.5mL=12g�������ɻ���ϩ��������Ϊm�������ݷ���ʽ�ã�
100 82
12g m
��m=![]() =9.84g����ʵ�����õ��Ļ���ϩ����Ϊ6.5g�������Ϊ��
=9.84g����ʵ�����õ��Ļ���ϩ����Ϊ6.5g�������Ϊ��![]() ��100%=66.1%���ʴ�Ϊ�����ڣ�66.1%��
��100%=66.1%���ʴ�Ϊ�����ڣ�66.1%��

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�����Ŀ���������Լ����͡��ձ��е����������������ʵ��Ŀ�ġ�����


ʵ��Ŀ�� | �Լ� | �ձ��е����� | |
A | ���������ǿ�ڱ��� | ̼��������Һ | �ٴ��� �ڱ�����Һ |
B | �ǻ��Ա����Ļ�����Ӱ�� | ������ˮ | �ٱ� �ڱ�����Һ |
C | ���Ա����Ļ�����Ӱ�� | ���Ը��������Һ | �ٱ� �ڼױ� |
D | �Ҵ��ǻ��е���ԭ�Ӳ���ˮ�����е���ԭ�ӻ��� | ������ | ��ˮ ���Ҵ� |
A. A B. B C. C D. D