��Ŀ����
����Ŀ��ȼú�����Ṥҵβ���е�SO2����Ҫ�Ĵ�����Ⱦ����������·�������β���е�SO2��
����һ��ͨ������̿���û���
2C��s��+2SO2��g��![]() S2��g��+2CO2��g��
S2��g��+2CO2��g��
��1��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ _____________________������ij�ܱ������н���������Ӧ��Ϊ�˼ӿ�����SO2���ɲ�ȡ�Ĵ�ʩ��_____________________________________��
����������������������Һ��������
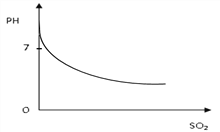
��2����β��(����������)����ͨ�뵽��������������Һ�У��������������ƣ�pHֵ�ı仯��ͼ��ʾ�������ͼʾ������PHֵ�仯��ԭ��_______________________________________��
��������������������Ķ��������ڻ���̿���������·�Ӧ��ȡ�Ȼ��������Ȼ�������SO2Cl2����Ҫ�����Ȼ���������һ����ɫҺ�壬�۵��C54.1�����е�69.1����
SO2(g)��Cl2(g)![]() SO2Cl2(l)+97.3kJ
SO2Cl2(l)+97.3kJ
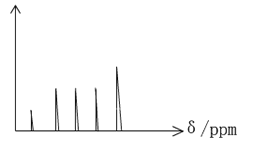
��3������Ӧ���������Ϊ2L��2������Һ������������2.7�ˣ��ö��������ʾ���������ڵĻ�ѧ��Ӧ����______________________��
��4�����ڴ������£����SO2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��____________________________________����_________________________________________ʱ˵��������Ӧ�Ѵﵽƽ��״̬��
���𰸡� 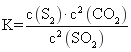 �����¶Ȼ�����ѹǿ��ʹ���ʵ������� ����������Һ����ˮ��ʼ���(PH>7)����ͨ�����������������������������ƣ���HSO3-���루��������ԣ��̶ȴ�����ˮ�⣨ˮ��ʼ��ԣ��̶ȣ�������Һ�����ԡ����Ŷ��������ͨ�룬��������Ũ����С������������Ũ��������һֱ����Ӧ��ȫ����PHֵ��Сֱ������ 0.005mol/(L��min) �����¶Ȼ�����ѹǿ ��������ɫ���ٸı��Һ���������ٸı�ȵ�
�����¶Ȼ�����ѹǿ��ʹ���ʵ������� ����������Һ����ˮ��ʼ���(PH>7)����ͨ�����������������������������ƣ���HSO3-���루��������ԣ��̶ȴ�����ˮ�⣨ˮ��ʼ��ԣ��̶ȣ�������Һ�����ԡ����Ŷ��������ͨ�룬��������Ũ����С������������Ũ��������һֱ����Ӧ��ȫ����PHֵ��Сֱ������ 0.005mol/(L��min) �����¶Ȼ�����ѹǿ ��������ɫ���ٸı��Һ���������ٸı�ȵ�
���������������������ͨ������SO2��Ⱦ�ķ������ۣ���Ҫ���黯ѧƽ�ⳣ������ʽ����ѧƽ���ƶ�������ˮ�⼰������ʵĵ��롢��ѧ��Ӧ���ʼ���Ȼ���֪ʶ�����鿼����ʶͼ��������ѧ�������������û�ѧƽ��ԭ�����ʵ�������������
��������1��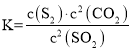 ���ӿ�����SO2�����跨��߷�Ӧ���ʣ����������һ��ʱ�����Բ�ȡ�Ĵ�ʩ������߷�Ӧ��ϵ���¶ȡ���߷�Ӧ���Ũ�ȡ�ʹ�ú��ʵĴ����ȡ���ȷ�𰸣�
���ӿ�����SO2�����跨��߷�Ӧ���ʣ����������һ��ʱ�����Բ�ȡ�Ĵ�ʩ������߷�Ӧ��ϵ���¶ȡ���߷�Ӧ���Ũ�ȡ�ʹ�ú��ʵĴ����ȡ���ȷ�𰸣� 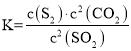 ����߷�Ӧ��ϵ���¶ȣ�������߷�Ӧ���Ũ�ȡ�ʹ�ú��ʵĴ���������2��SO2ͨ�뱥�͵�Na2SO3��Һ��ʱ��Na2SO3+SO2+H2O=2NaHSO3��Na2SO3��Һ������SO32-ˮ����Һ�ʼ��ԣ���Ӧ���ɵ�NaHSO3������HSO3-�Ե���Ϊ������������SO2����ͨ�룬��Һ��Na2SO3��ת���NaHSO3����Һ��pHֵ��С�������ȶ�����ȷ�𰸣�����������Һ����ˮ��ʼ���(PH>7)����ͨ�����������������������������ƣ���HSO3-���루��������ԣ��̶ȴ�����ˮ�⣨ˮ��ʼ��ԣ��̶ȣ�������Һ�����ԡ����Ŷ��������ͨ�룬��������Ũ����С������������Ũ��������һֱ����Ӧ��ȫ����pHֵ��Сֱ����������3��
����߷�Ӧ��ϵ���¶ȣ�������߷�Ӧ���Ũ�ȡ�ʹ�ú��ʵĴ���������2��SO2ͨ�뱥�͵�Na2SO3��Һ��ʱ��Na2SO3+SO2+H2O=2NaHSO3��Na2SO3��Һ������SO32-ˮ����Һ�ʼ��ԣ���Ӧ���ɵ�NaHSO3������HSO3-�Ե���Ϊ������������SO2����ͨ�룬��Һ��Na2SO3��ת���NaHSO3����Һ��pHֵ��С�������ȶ�����ȷ�𰸣�����������Һ����ˮ��ʼ���(PH>7)����ͨ�����������������������������ƣ���HSO3-���루��������ԣ��̶ȴ�����ˮ�⣨ˮ��ʼ��ԣ��̶ȣ�������Һ�����ԡ����Ŷ��������ͨ�룬��������Ũ����С������������Ũ��������һֱ����Ӧ��ȫ����pHֵ��Сֱ����������3��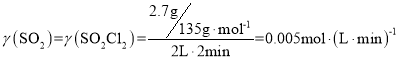 ����ȷ�𰸣�0.005mol/(L��min)����4�����SO2��ת���ʵĴ�ʩ�Ǵ�ʹƽ�������ƶ����ӷ�Ӧ���ȿ��Բ��ý��µķ�����ʹƽ�������ƶ����ӷ�Ӧǰ�����������С���������Բ��ü�ѹ�ķ�����ʹƽ�������ƶ�����ʵ������Ч����������Ӧ����ƽ�����ϵ��c(Cl2)���ֲ�����ɫ���䡢Һ����������ֲ��䡣��ȷ�𰸣������¶Ȼ�����ѹǿ����������ɫ���ٸı��Һ���������ٸı䡣
����ȷ�𰸣�0.005mol/(L��min)����4�����SO2��ת���ʵĴ�ʩ�Ǵ�ʹƽ�������ƶ����ӷ�Ӧ���ȿ��Բ��ý��µķ�����ʹƽ�������ƶ����ӷ�Ӧǰ�����������С���������Բ��ü�ѹ�ķ�����ʹƽ�������ƶ�����ʵ������Ч����������Ӧ����ƽ�����ϵ��c(Cl2)���ֲ�����ɫ���䡢Һ����������ֲ��䡣��ȷ�𰸣������¶Ȼ�����ѹǿ����������ɫ���ٸı��Һ���������ٸı䡣

 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�����Ŀ��ij��ѧС�������ͼ��ʾ��װ�ã��û������Ʊ�����ϩ��
��֪��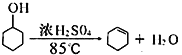
��Է������� | �ܶ�/g cm-3 | �۵�/�� | �е�/�� | �ܽ��� | |
������ | 100 | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 82 | 0.81 | -103 | 83 | ������ˮ |
��1���Ʊ���Ʒ
��12.5 mL��������1mLŨ��������Թ�A�У�ҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
�����Թ��л�ϻ��Ѵ���Ũ�������ʱ������ҩƷ���Ⱥ�˳��Ϊ______________________��
���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ������_________������ĸ����
A.�������� B.��ȴ�� C.���貹�� D.��������
�۽��Թ�C���ڱ�ˮ�е�Ŀ����_______________________________________________��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����Ʒ�м��뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_______��(����������������)����Һ����________������ĸ��ϴ����
a.����KMnO4��Һ b.ϡ���� c.Na2CO3��Һ
���ٽ��ᴿ��Ļ���ϩ����ͼ��ʾװ�ý�������ͼ������a ��������_______________,ʵ������ȴˮ��______������ĸ���ڽ���������ʱҪ������ʯ�ң�Ŀ����__________________________��
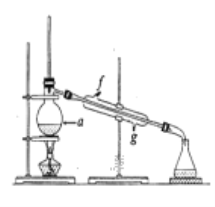
��3�������Ʊ���Ʒʱ���Ѵ����Ʒһ����������ʵ���ƵõĻ���ϩ��Ʒ����_______(�������������� ������)���۲�������ʵ�����õ��Ļ���ϩ����Ϊ6.5g���������______������һλС������
����Ŀ���ϳɰ��ķ�ӦΪN2��3H2![]() 2NH3��Q���±���ij�η�ӦʱNH3��Ũ�ȱ仯��
2NH3��Q���±���ij�η�ӦʱNH3��Ũ�ȱ仯��
ʱ�䣨min�� | 0 | 5 | 10 | 15 | 20 |
c(NH3)��mol/L�� | 0 | 0.30 | 0.44 | 0.50 | 0.50 |
���������գ�
��1���ɱ������ݼ���0��10 minʱ����H2��ʾ��ƽ����Ӧ����Ϊ________________�������¶ȣ���Ӧ��ƽ�ⳣ����______________������������������С������������������Ҫ����Ӧ���������H2�����ʣ��ɲ�ȡ�Ĵ�ʩ��________________��
��2������Ӧ������������ܶȱ��ֲ��䣬�ܷ�˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����˵�����ɡ�____________________________________________________________________
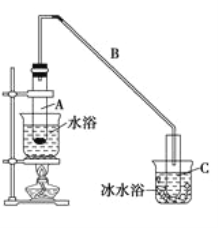
��3������ͼ��ʾװ�ý����йذ������ʵ�ʵ�飬������ͷ�ι��е�ˮ����ֹˮ�к��ʵ��������_____________��

��4����������ˮ����Һ�Լ��Ե�ԭ���ǣ��÷���ʽ��ʾ��____________________________________________�����ڰ�ˮ����εμ����ᣬ����Һ��pH��__________������������������С������������������ǡ����ȫ��Ӧʱ����c(NH4��)________c(Cl��)������������������������������
����Ŀ�����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
Ԫ�ط��� | Ԫ�����ʻ�ԭ�ӽṹ |
T | M���ϵĵ�������ԭ�Ӻ�����Ӳ�����2�� |
X | �����������Ǵ�����������2�� |
Y | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
Z | Ԫ�����������+7�� |
���������в���ȷ���ǣ� ��
A.14X������ⶨ�������
B.Z�ķǽ����Ա�Tǿ
C.Y��Tλ��ͬһ����
D.XԪ���γɵĻ���������ܶ�