��Ŀ����
��ҵ�Ͽ������÷����е�CO2Ϊԭ����ȡ�״����䷴Ӧ����ʽΪ��CO2+3H2 CH3OH+H2O����ش��������⣺
CH3OH+H2O����ش��������⣺
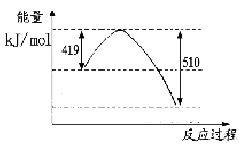
��1����֪���³�ѹ�����з�Ӧ�������仯����ͼ��ʾ��
|
|
|
|

 д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ__ _��
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ__ _���÷�Ӧ�ġ�S____0(�>����<����=��)���� ______����������ڸ÷�Ӧ�Է����С�
��2�����������Ӧ����ʽ��ƽ�ⳣ��Kֵ�����÷�Ӧ__ ��ѡ���ţ���
A��һ��������Ӧ�����ƶ� B����ƽ���ƶ�ʱ����Ӧ������������С
C��һ�����淴Ӧ�����ƶ� D����ƽ���ƶ�ʱ�淴Ӧ�����ȼ�С������
��3�����������Ӧ�����������ܱ������з�������˵����Ӧ�Ѵﵽƽ��״̬���� __ ��ѡ���ţ���
A��3v��(H2)=v��(CO2) B��C(H2) = C(CO2)
C��������������ܶȲ��� D��������ѹǿ����
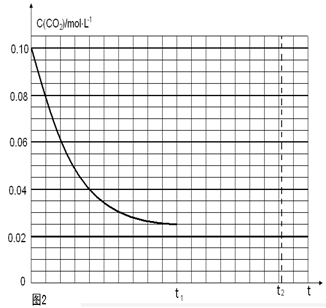
��4������Ӧ�������ݻ�Ϊ2.0L����Ӧʱ��4.0 min��������������ܶ�������2.0g/L�������ʱ����CO2��ƽ����Ӧ����Ϊ ����Ӧ��t1ʱ�ﵽƽ�⣬������c(CO2)��ʱ��t�仯����������ͼ��ʾ�����������������䣬t1ʱ���������ѹ����1L���뻭��t1��c(CO2)��ʱ��t�仯��������(t2�ﵽ�µ�ƽ��)��
��1��3H2(g) + CO2(g)= CH3OH(l)+ H2O(l)����H=��50KJ/mol��<�� ���£���2��A��D��
��3��CD����4��0.01mol.L-1.min-1����ͼ
���������������1����������ɵ��Ȼ�ѧ����ʽ����CO(g)+H2O(l)=CO2(g)+H2(g) ��H=��41KJ/mol; ��CO(g) +H2(g) =CH3OH(l)��H=��91KJ/mol; �ڣ��٣������ɵ�3H2(g) + CO2(g)= CH3OH(l)+ H2O(l)����H=��50KJ/mol;�ɷ���ʽ��֪���÷�Ӧ��һ����ϵ�Ļ��ҳ̶ȼ�С�ķ�Ӧ�����ԡ�S<0�����ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ�����Է�Ӧ�ڵ�������������ڸ÷�Ӧ�Է����С���2�����ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ������������Ӧ����ʽ��ƽ�ⳣ��Kֵ�����ƽ�������ƶ�������Kֻ���¶��йأ�����ѹǿ��Ũ�ȵ��أ�����ֻ���¶Ƚ��Ͳſ�������������������ʱ��V����V�涼��С��V���С�Ķ࣬V��>V�棬ƽ�������ƶ����淴Ӧ�����ȼ�С�����������ӡ����ѡ��ΪA��D����3��A������Ӧ�ﵽƽ�⣬��v��(H2)= 3v��(CO2)������B�����ڶ�������ʱ�ǰ���3:1�����ʵ����Ĺ�ϵ���ĵģ������ڿ�ʼ���������������ֻ�а���ijһȷ���ı�����ϣ��ﵽƽ��ʱ���й�ϵ��C(H2) = C(CO2)����˲�����Ϊ�ж�ƽ��ı�־������C��������������Һ̬���ʣ�����Ӧδ�ﵽƽ�⣬������������ͻᷢ���仯��������ܶ�Ҳ�ᷢ���ı䡣���������������ܶȲ��䣬������Ϊ�ж�ƽ��ı�־����ȷ��D���÷�Ӧ�Ƿ�Ӧǰ������������ȵķ�Ӧ�����δ�ﵽƽ�⣬����������ʵ����ͻᷢ���仯���������������ѹǿ�ͻ�ı䡣���������ѹǿ���������Ϊ�ж�ƽ��ı�־����ȷ����4����Ӧ�������ݻ�Ϊ2.0L��������������ܶ�������2.0g/L�������������������2.0g/L��2.0L=4.0g.�������ӵ�CO2������Ϊ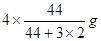 �����Ԧ�n(CO2)=" ��m��M="
�����Ԧ�n(CO2)=" ��m��M="  =0.08mol,���V(CO2)= ��c(CO2)�¦�t="(0.08mol" ��2L)��4min = 0.01mol/(L��min)��
=0.08mol,���V(CO2)= ��c(CO2)�¦�t="(0.08mol" ��2L)��4min = 0.01mol/(L��min)��
��ͼ���֪����ӦCO2(g)+3H2(g) CH3OH(l)+H2O(l)����ʼʱc(CO2)=0.10mol/L��ƽ��ʱc(CO2)=0.025mol/L����c(CO2)=0.075mol/L������ƽ��ʱ�����ʵ�Ũ��Ϊ��c(H2)=0.075mol/L���÷�Ӧ��ƽ�ⳣ��
CH3OH(l)+H2O(l)����ʼʱc(CO2)=0.10mol/L��ƽ��ʱc(CO2)=0.025mol/L����c(CO2)=0.075mol/L������ƽ��ʱ�����ʵ�Ũ��Ϊ��c(H2)=0.075mol/L���÷�Ӧ��ƽ�ⳣ��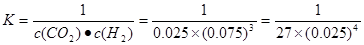 �������������������䣬t1ʱ���������ѹ����1L��c(CO2)=0.05mol/L��c(H2)=0.15mol/L��ƽ�������ƶ���������c(CO2)=xmol/L,������c(H2)=3xmol/L��ƽ��ʱ�����ʵ�Ũ�ȷֱ�Ϊc(CO2)="(0.05-x)mol/L" ��c(H2)="(0.15-3x)mol/L" ��ƽ�ⳣ�����䡣��
�������������������䣬t1ʱ���������ѹ����1L��c(CO2)=0.05mol/L��c(H2)=0.15mol/L��ƽ�������ƶ���������c(CO2)=xmol/L,������c(H2)=3xmol/L��ƽ��ʱ�����ʵ�Ũ�ȷֱ�Ϊc(CO2)="(0.05-x)mol/L" ��c(H2)="(0.15-3x)mol/L" ��ƽ�ⳣ�����䡣��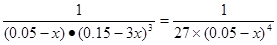 �����x= 0.025mol/L����t2�ﵽ�µ�ƽ��ʱc(CO2)=0.025mol/L.��t1��c(CO2)��ʱ��t�仯��������Ϊ��ͼ��ʾ��
�����x= 0.025mol/L����t2�ﵽ�µ�ƽ��ʱc(CO2)=0.025mol/L.��t1��c(CO2)��ʱ��t�仯��������Ϊ��ͼ��ʾ��
���㣺�����Ȼ�ѧ����ʽ����д����ѧƽ��״̬���жϡ���Ӧ�ķ����ԡ���ѧ��Ӧ���ʵļ��㡢CO2Ũ����ʱ��ͼ��ı�ʾ��

�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����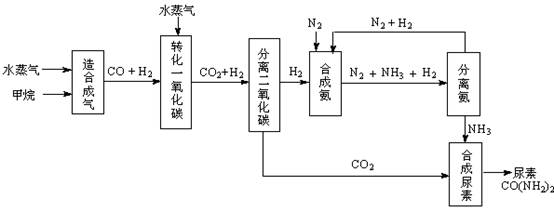
��1������ϳ������������Ȼ�ѧ����ʽ��CH4(g)+H2O(g) 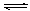 CO(g)+3H2(g)����H��0
CO(g)+3H2(g)����H��0
�ں��º��ݵ������£������CH4�ķ�Ӧ���ʺ�ת���ʣ����д�ʩ���е��� ��
A������ѹǿ B�������¶� C������He�� D������ˮ����Ũ��
��2����ת��һ����̼�������ķ���ʽ��H2O(g) +CO(g) 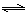 H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
�����̼��[ n��H2O��/n��CO��]��Kֵ ������������䡱��С���������÷�Ӧ��400��ʱ���У���ʼͨ������ʵ�����H2O��CO����Ӧ���е�ijһʱ��ʱCO��CO2��Ũ�ȱ�Ϊ1��3����ʱv������ v���棩�����������������������
��3���йغϳɰ���ҵ��˵������ȷ���� ��
A���÷�Ӧ�����˹��̵�
B���ϳɰ���ҵ��ʹ�ô�������߷�Ӧ���������
C���ϳɰ���Ӧ�¶ȿ�����500�����ң�Ŀ����ʹ��ѧƽ��������Ӧ�����ƶ�
D���ϳɰ���ҵ����ѭ����������Ҫԭ����Ϊ�˼ӿ췴Ӧ����
��4���������ع����У�������n(NH3)��n(CO2)��������Ϊ ����ʵ�����������У�����ʹn(NH3)��n(CO2)��3��������Ϊ ��
��5��������ϳɰ�����ת����Ϊ60��ʱ����3.0��108 L����Ϊԭ���ܹ��ϳ� L ������������������ڱ�״���²ⶨ��
��16�֣��������ƣ�Na2FeO4�����к�ǿ�������ԣ��㷺Ӧ���ھ�ˮ����ع�ҵ�������Դ�FeO(����CuO��Al2O3��SiO2������)�Ʊ��������Ƶ������������£��ش��������⣺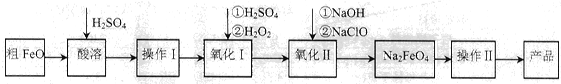
��֪��NaClO���ȶ��������ֽ⡣
��1����FeO���ܹ�����ͨ��ˮ���������£�����Ŀ����__________________________��
��2������IĿ���ǵõ��ߴ���FeSO4��Һ��������I�з�Ӧ�����ӷ���ʽΪ_________��
��3������������Ҫ��Ũ��NaClO��Һ������Cl2��NaOH��Һ��Ӧ�Ʊ�
��Cl2��NaOH��Һ��Ӧ�����ӷ���ʽΪ_________________��
���ڲ�ͬ�¶��½��и÷�Ӧ����Ӧ��ͬһ��ʱ��������NaClOŨ�����£�
| �¶�/�� | 15 | 20 | 25 | 30 | 35 | 40 | 45 |
| NaClOŨ��/mol��L-1 | 4.6 | 5.2 | 5.4 | 5.5 | 4.5 | 3.5 | 2 |
��ԭ��Ϊ____________________________________________________________________��
��4����ҵҲ���õ�ⷨ�Ʊ�Na2FeO4����ԭ��ΪFe+2OH-+2H2O���FeO42-+3H2�����������в�����Ƶ��ز��ڴ���ķ����ڻ�����װ�á�

��ѡ���ϣ���Ƭ��ͭƬ��̼����ŨNaOH��Һ��ŨHCl��
��������ӦʽΪ��________________________________��
�״�����Ϊȼ�ϵ�ص�ԭ�ϡ���CH4��H2OΪԭ�ϣ�ͨ�����з�Ӧ���Ʊ��״���
I��CH4(g)+H2O(g) CO(g)+3H2(g) ��H=+206.0kJ?mol��1
CO(g)+3H2(g) ��H=+206.0kJ?mol��1
II��CO(g)+2H2(g) CH3OH(g) ��H=��129.0kJ?mol��1
CH3OH(g) ��H=��129.0kJ?mol��1
��1��CH4(g)��H2O(g)��Ӧ����CH3OH(g)��H2(g)���Ȼ�ѧ����ʽΪ ��
��2����1.0mol CH4��1.0mol H2O(g)ͨ���ݻ�Ϊ100 L�ķ�Ӧ�ң���һ�������·�����ӦI�������һ����ѹǿ��CH4��ת�������¶ȵĹ�ϵ��ͼ��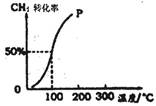
�ټ���100��ʱ�ﵽƽ�����蹹ʱ��Ϊ5min������H2��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ ��
��1000Cʱ��ӦI��ƽ�ⳣ��Ϊ ��
��3����ѹǿΪ0.1 MPa���¶�Ϊ300�������£���a molCO��2a mol H2�Ļ�������ڴ��������·�����ӦII���ɼ״���ƽ����������ݻ�ѹ����ԭ����1/2�������������䣬��ƽ����ϵ������Ӱ���� ������ĸ��ţ���
| A��ƽ�ⳣ��K���� | B������Ӧ���ʼӿ죬�淴Ӧ���ʼ��� |
| C��CH3OH�����ʵ������� | D������ƽ��c(H2)/c(CH3OH)��С |

 �ų����ݺ�������������MnO
�ų����ݺ�������������MnO �ܿ�������壺________��
�ܿ�������壺________�� zC�ﵽƽ�⣺
zC�ﵽƽ�⣺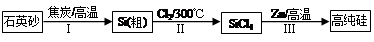
 Si(S)+2ZnCl2(l) ��H1��0
Si(S)+2ZnCl2(l) ��H1��0 N2O4(g)����H��-a kJ��mol��1 (a>0) ��N2O4�����ʵ���Ũ����ʱ��仯��ͼ����ƽ��ʱ�� N2O4��Ũ��ΪNO2��2�����ش��������⡣
N2O4(g)����H��-a kJ��mol��1 (a>0) ��N2O4�����ʵ���Ũ����ʱ��仯��ͼ����ƽ��ʱ�� N2O4��Ũ��ΪNO2��2�����ش��������⡣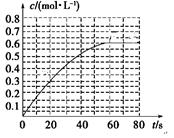
 2C(g)+xD(g)������5 min��ﵽ��ѧƽ�⣬��ʱ����2 molC����֪�ڴ�ʱD��ƽ������Ϊ0��15 mol��L-1��min-1��
2C(g)+xD(g)������5 min��ﵽ��ѧƽ�⣬��ʱ����2 molC����֪�ڴ�ʱD��ƽ������Ϊ0��15 mol��L-1��min-1��