��Ŀ����
A��B��X��Y��Z��ԭ���������ε����Ķ�����Ԫ�أ�����A��Yͬ���壬X��Zͬ���壬A��B��A��X�����γ�10�����ӻ����B��Z������������֮��Ϊ2�U3������������Y2X2��ˮ��Ӧ����X�ĵ��ʣ�����Һ��ʹ��̪��Һ��졣��ش��������⡣
��1�� X�����ڱ��е�λ����__________________________
��2�� ������Y2X2�ĵ���ʽΪ _________ �������еĻ�ѧ�������� _________
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
��3�� A��X��A��Z�����γ�18�����ӵĻ���������ֻ��������Ӧ����Z�Ļ�ѧ����ʽΪ_____________________________________��
��4�� A�ĵ�����X�ĵ��ʿ��Ƴ����͵Ļ�ѧ��Դ��KOH��Һ���������Һ���������缫���ɶ����̼�Ƴɣ�ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ磬���缫��ӦʽΪ_____________________________________��
��5�� д��������Y2X2��ˮ��Ӧ�����ӷ���ʽ_____________________��
��6�� B�����������ĽṹʽΪ_______________________________��
��1���ڶ����ڢ�A��
��2�� Na2O2 �ĵ���ʽ Na+[:O:O:]2-Na+
�� A��C
��3��H2O2+H2S=S��+2H2O
��4��H2 �C 2e- + 2OH- == 2H2O��2H2 �C 4e- + 4OH- == 4H2O
��5��2Na2O2��2H2O===4 Na����4OH-��O2��
��6�� O=C=O
��������������ɳ���������Y2X2��ˮ��Ӧ����X�ĵ��ʣ�����Һ��ʹ��̪��Һ����֪��XΪ����YΪ�ơ�����A��B��A��X�����γ�10�����ӻ������֪AΪ�⡣����X��Zͬ����˵��ZΪ��B��Z������������֮��Ϊ2�U3��֪��B����������Ϊ4������BΪ̼��
���㣺����Ԫ�����ڱ���Ԫ�������ɵ����֪ʶ��

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д������й�ԭ�ӽṹ��Ԫ�������ɵı�������ȷ���ǣ� ��
��ԭ������Ϊ16��Ԫ�ص�����ϼ�Ϊ+4
��IA��Ԫ����ͬ������ʧ����������ǿ��Ԫ��
�۵�2����IVA��Ԫ�ص�ԭ�Ӻ˵������������һ��Ϊ6
��ԭ������Ϊ13��Ԫ��λ��Ԫ�����ڱ��ĵ�3����III��
| A���٢� | B���٢� | C���ڢ� | D���ۢ� |
��10�֣�A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȡ�
| A | | C |
| | B | |
��1��A��B��C����Ԫ�ص����Ʒֱ�Ϊ �� �� ��
��2��Bλ��Ԫ�����ڱ��е� ���ڡ��� �塣
��3��C��ԭ�ӽṹʾ��ͼΪ ��C�ĵ�����
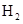 ��Ӧ�Ļ�ѧ��
��Ӧ�Ļ�ѧ�� ��ʽΪ ��
��4��д��A����̬�⻯����B������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ ��
��10�֣��±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | | �� | �� | �� |
| 4 | �� | | | | | | | |
��2��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��_______��������ǿ�Ļ�����ĵ���ʽ��:_____________��
��3������������������������Ԫ����_____________��д���������������������Ʒ�Ӧ�����ӷ���ʽ____________________________________________��
��4���õ���ʽ��ʾԪ�آ�����γɵĵĻ�������γɹ���_________________________ ���û���������__________________(�� �����ۡ������ӡ�)�����
��5��Ԫ�آ�����γɵĵĻ�����ĵ���ʽΪ______________________ ���û���������___________ ������ԡ����Ǽ��ԡ������γɵġ�
��10�֣��±�ΪԪ�����ڱ��е�һ���֣��û�ѧʽ��Ԫ�ط��Żش��������⣺
| | IA | ��A | ��A | ��A | VA | ��A | ��A | 0 |
| 2 | | | | �� | | �� | | |
| 3 | �� | �� | �� | | | | �� | �� |
| 4 | �� | �� | | | | | �� | |
��2���ڢ٢ڢݵ�����������ˮ�����У�������ǿ����__________���ѧʽ����
��3��Ԫ�آߵij������⻯��Ļ�ѧʽΪ__ __�����⻯�ﳣ���º�Ԫ�آڵĵ��ʷ�Ӧ�����ӷ���ʽ�ǣ�_______________________�����⻯����Ԫ�آ�ĵ��ʷ�Ӧ�����ӷ���ʽ��___________________________��
��4���ٺ͢������������Ӧ��ˮ���ﻯѧʽ�ֱ�Ϊ___________��_______��
��5���ٺ͢�����������Ӧ��ˮ�������Ӧ�Ļ�ѧ����ʽΪ__________________
��6�������ֱ���H2�γɵ��⻯����ȶ��ԣ�__________�����û�ѧʽ��ʾ������������Ӧ��ˮ��Һ�����ԣ�_______________�����û�ѧʽ��