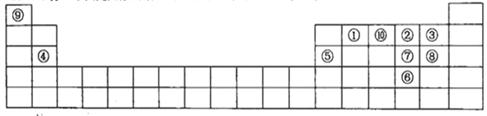
��Ŀ����
��11�֣�ԭ��������������Ķ�����Ԫ��A��B��C��D��E��F������ A��B����������C��D�������ӵĵ����Ų�ʽ��Ϊls22s22p6��Aԭ�Ӻ�����2��δ�ɶԵ��ӣ�C���ʿ�����ˮ��Ӧ����������ˮ��Ӧ��E��Fԭ���ڻ�̬ʱ�����ӵĹ����9������Eԭ�Ӻ�����3��δ�ɶԵ��ӣ�F���γ���A��ͬ��̬�������ӣ���A���Ӱ뾶С��F���ӡ��ش�
��1��BԪ�ص�����Ϊ ��
��2��A��F���γ�������ѧ��ѧ�����Ļ���������ʽ�ֱ�Ϊ �� �����ӿռ乹�ͷֱ�Ϊ ��
��3����д����̬ԭ��E�ļ۵����Ų�ʽ
��4����������Ԫ�ذ��縺�ԴӴ�С�������� ��дԪ�ط��ű�ʾ����
��5��C��D��E��FԪ�ص�һ�����ܴ�С����������� ��дԪ�ط��ţ���
��6��A��B��C��D����Ԫ�صļ����Ӱ��뾶��С�����˳��Ϊ�������ӷ��ű�ʾ��__________________________��
��7��C��A�γɵĻ��������� ���壬�˻������NaCl������Ƚ��۵�ĸߵ�ϵΪ
���û�ѧʽ��ʾ��С��ϵ����ԭ���� ��
��1����
��2��SO2��SO3��V�͡�ƽ��������
��3��3s23p3
��4��F>O>S>P>Al>Mg
��5��Al<Mg<S<P
��6��Al3+<Mg2+<F<O2-
��7�����ӣ�MgO>NaCl��MgO�ľ����ܴ���NaCl�ľ�����
�������������A����������10�����ӣ���A��ԭ������2��δ�ɶԵ��ӣ�����A��������������6����A��OԪ�أ�Bֻ����FԪ�أ�C���ʿ�����ˮ��Ӧ����������ˮ��Ӧ����C�������ӵĺ�����10�����ӣ�����C��MgԪ�أ���D��AlԪ�أ�Eԭ���ڻ�̬ʱ�����ӵĹ����9�����ֱ���1s��2s��2p��3s��3p����Eԭ�Ӻ�����3��δ�ɶԵ��ӣ�˵��3p�����3�����ӣ�����E��15��Ԫ��P��F���γ���A��ͬ��̬�������ӣ�˵��F��A��ͬ����Ԫ�أ���A���Ӱ뾶С��F���ӣ�����������Aͬ�����Ԫ����S������F��SԪ�ء�
BԪ�ص������Ƿ���
O��S�γɵĻ�����Ļ�ѧʽ�ֱ���SO2��SO3��ǰ�߷�����Sԭ�ӵļ۲���Ӷ�����3����һ�Թ¶Ե��ӣ����Կռ乹����V�ͣ���SO3�����е�Sԭ�ӵļ۲���Ӷ�����3���¶Ե��ӣ����Կռ乹����ƽ�������Σ�
E��15��Ԫ��P���۵�������5�����Լ۲�����Ų�ʽΪ3s23p3
��4������Ԫ�������ɣ�ͬ����Ԫ�ش����ҵ縺��������ͬ����Ԫ�ش��ϵ��µ縺����������������Ԫ�صĵ縺�ԴӴ�С��������F>O>S>P>Al>Mg��
��5������Ԫ�������ɣ�ͬ����Ԫ�ش�����Ԫ�صĵ�һ������������Mg�������3s�����ȫ����״̬���Ƚ��ȶ���ʹ��һ����������P���������ӵ�3p����ǰ����״̬���Ƚ��ȶ������Ե�һ�����ܱ�S����C��D��E��FԪ�ص�һ�����ܴ�С�����������Al<Mg<S<P
��6�����ӵİ뾶��Ҫ�ɵ��Ӳ�������������Ǻ˵������A��B��C��D����Ԫ�صļ����ӵĵ��Ӳ�����ͬ���˵����Խ�����Ӱ뾶ԽС������A��B��C��D����Ԫ�صļ����Ӱ��뾶��С�����˳��ΪAl3+<Mg2+<F<O2-
��7��O��Mg�γɵĻ������������ӻ���������γɵľ��������Ӿ��壻MgO�е����Ӱ뾶С��NaCl�е����Ӱ뾶����������ɽ϶࣬����MgO�ľ����ܽϴ��۵����NaCl��
���㣺����Ԫ�ص��ƶϣ�Ԫ�������ɵ�Ӧ�ã����ӿռ乹�͡����ʵ��ж�

 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�������������˵������A�϶��Ƚ���B�Ļ�����ǿ���ǡ��� ��
| A��Aԭ�ӵ�������������Bԭ�ӵ������������� |
| B��Aԭ�ӵ��Ӳ�����Bԭ�ӵ��Ӳ����� |
| C��1mol A�������û�����H2��1molB�������û���H2�� |
| D������ʱ��A�ܴ�ϡ�������û����⣬��B���� |
����˵����ȷ����(����)
| A��Ԫ��ԭ�ӵ���������������Ԫ�ص�����ϼ� |
| B�������ԭ���У�����˽Ͻ����������˶��ĵ��������ϸ� |
| C��Ԫ�����ڱ���λ�ڽ����ͷǽ����ֽ��߸�����Ԫ�����ڹ���Ԫ�� |
| D��Na��Mg��Alʧ��������������������Ӧˮ����ļ��Ծ����μ��� |
��8��ÿ��1�֣�
��1������3�ֲ�ͬ����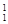 H��
H��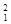 H��
H�� H��ʾ______��Ԫ�أ�______�ֺ��أ�
H��ʾ______��Ԫ�أ�______�ֺ��أ�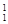 H��
H��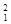 H��
H�� H����Ϊ__________________��
H����Ϊ__________________��
��2�����и��������У�����Ϊͬ���칹�����( )
| A��ˮ��� | B��O2��O3 |
C��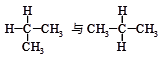 | D�� |
��3��K2O�� ����4��CO2�� ��