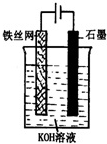
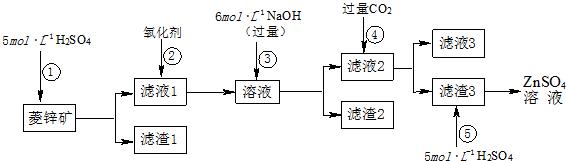
��Ŀ����
1������A��B��C��D����Ԫ�أ�ǰ����Ԫ�ص����ӽṹ������ԭ�Ӿ�����ͬ�ĺ�������Ų���Aû������̬�Ļ����B��D���γɵ�D2B���ӣ�0.2mol��Cԭ���ܴ������û���2.24LH2����״���£���D��ԭ�Ӻ���û�����ӣ���1�����������������ƶ�B��D��Ԫ�����ƣ�B������D���⣮
��2���õ���ʽ��ʾD2B��������γɹ��̣�
 ��
����3��C2B2�ĵ���ʽ
 ���������ӻ��������ӻ�����������ۻ������
���������ӻ��������ӻ�����������ۻ��������4��д��A������D2B��Ӧ�Ļ�ѧ����ʽ2 F2+2H2O�T4HF+O2 ��
���� A��B��C��D����Ԫ�أ�ǰ����Ԫ�ص����ӽṹ������ԭ�Ӿ�����ͬ�ĺ�������Ų���0.2mol��Cԭ���ܴ������û�����2.24L H2������£�����ZΪ�������仯�ϼ�=$\frac{\frac{2.24L}{22.4L/mol}��2}{0.2mol}$=1������֪ZΪNa��D��ԭ�Ӻ���û�����ӣ���DΪHԪ�أ�Aû������̬�Ļ����B��D���γɵ�D2B���ӣ���AΪFԪ�ء�BΪOԪ�أ��ݴ˽��
��� �⣺A��B��C��D����Ԫ�أ�ǰ����Ԫ�ص����ӽṹ������ԭ�Ӿ�����ͬ�ĺ�������Ų���0.2mol��Cԭ���ܴ������û�����2.24L H2������£�����ZΪ�������仯�ϼ�=$\frac{\frac{2.24L}{22.4L/mol}��2}{0.2mol}$=1������֪ZΪNa��D��ԭ�Ӻ���û�����ӣ���DΪHԪ�أ�Aû������̬�Ļ����B��D���γɵ�D2B���ӣ���AΪFԪ�ء�BΪOԪ�أ�
��1��������������֪��BΪ��Ԫ�أ�DΪ��Ԫ�أ��ʴ�Ϊ�������⣻
��2���õ���ʽ��ʾH2O��������γɹ��̣� ��
��
�ʴ�Ϊ�� ��
��
��3��C2B2Ϊ Na2O2���Ⱥ������Ӽ��ֺ��й��ۼ��������Ӿ��壬����ʽΪ ��
��
�� �����ӣ�
�����ӣ�
��4��F2��H2O��Ӧ�Ļ�ѧ����ʽΪ��2 F2+2H2O�T4HF+O2 ��
�ʴ�Ϊ��2 F2+2H2O�T4HF+O2 ��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ������ضԻ�ѧ����Ŀ��飬ע�����������õ���ʽ��ʾ��ѧ�������ʵ��γɣ�

| A�� | 2L HF����ֽ��1L H2��1L F2����270KJ���� | |
| B�� | 1mol H2��1mol F2��Ӧ����2molҺ̬HF�ų�������С��270KJ | |
| C�� | ����ͬ������1mol H2��1mol F2�������ܺʹ���2mol HF������� | |
| D�� | 1���������1�������ӷ�Ӧ����2����������ӷų�279KJ���� |
 �й�����ս����-31ʹ���˸�ǿ�ȡ����µ��ѺϽ���ϣ���ҵ��ұ���ѵķ�Ӧ���£�
�й�����ս����-31ʹ���˸�ǿ�ȡ����µ��ѺϽ���ϣ���ҵ��ұ���ѵķ�Ӧ���£�TiCl4+2Mg$\frac{\underline{\;����\;}}{\;}$Ti+2MgCl2�������йظ÷�Ӧ��˵����ȷ���ǣ�������
| A�� | TiCl4�ǻ�ԭ�� | B�� | Mg������ | ||
| C�� | TiCl4����������Ӧ | D�� | Mg�õ����� |
| A�� | ̼��������Һ�������ı���ʯ��ˮ��Ӧ2HCO3-+Ca2++2OH-�TCaCO3��+CO32-+2H2O | |
| B�� | ������������Һ�м���ϡ���ᡡOH-+H+�TH2O | |
| C�� | ���Ȼ�����Һ�м������ۡ�Fe+FeCl3=2Fe2++3Cl- | |
| D�� | Ca��ClO��2��Һ��ͨ������Ķ�����������Ca2++2ClO-+H2O+SO2�TCaSO3��+2HClO |
| A�� | ��10mL��Ͳ��ȡϡ������Һ 8.0mL | |
| B�� | �ø����pH��ֽ�ⶨ��ˮ��pH | |
| C�� | �ü�ʽ�ζ�����ȡKMnO4��Һ 19.60mL | |
| D�� | ʹ������ƿ������Һʱ������Һ�涨�ݺ�������Һ��Ũ��ƫ�� | |
| E�� | Բ����ƿ����ƿ�����������ʱ��Ӧ����ʯ������ |
| A�� | �������ɲ�����FeCl3��Һ���õ�Fe2O3���� | |
| B�� | ���ڳ�ʪ�Ļ��������� | |
| C�� | ������ˮ | |
| D�� | Ũ������Һ�г�ζ |