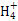ĢāÄæÄŚČŻ
Į×ĖįĢśļ®(LiFePO4)±»ČĻĪŖŹĒ×īÓŠĒ°Ķ¾µÄļ®Ąė×Óµē³ŲÕż¼«²ÄĮĻ”£Ä³ĘóŅµĄūÓĆø»Ģś½ž³öŅŗÉś³ÉĮ×ĖįĢśļ®,æŖ±ŁĮĖ“¦ĄķĮņĖįŃĒĢś·ĻŅŗŅ»ĢõŠĀĶ¾¾¶”£ĘäÖ÷ŅŖĮ÷³ĢČēĻĀ:
ŅŃÖŖ:H2TiO3ŹĒÖÖÄŃČÜÓŚĖ®µÄĪļÖŹ”£
(1)īŃĢśæóÓĆÅØĮņĖį“¦ĄķÖ®Ē°,ŠčŅŖ·ŪĖé,ĘäÄæµÄŹĒ ”””£
(2)TiO2+Ė®½āÉś³ÉH2TiO3µÄĄė×Ó·½³ĢŹ½ĪŖ ”””£
(3)¼ÓČėNaClO·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”””£
(4)ŌŚŹµŃéÖŠ,“ÓČÜŅŗÖŠ¹żĀĖ³öH2TiO3ŗó,ĖłµĆĀĖŅŗ»ė×Ē,Ó¦ČēŗĪ²Ł×÷”” ”£
(5)ĪŖ²ā¶ØīŃĢśæóÖŠĢśµÄŗ¬Įæ,ijĶ¬Ń§Č”¾ÅØĮņĖįµČ“¦ĄķµÄČÜŅŗ(“ĖŹ±īŃĢśæóÖŠµÄĢśŅŃČ«²æ×Ŗ»ÆĪŖ¶ž¼ŪĢśĄė×Ó),²ÉČ”KMnO4±ź×¼ŅŗµĪ¶ØFe2+µÄ·½·Ø:(²»æ¼ĀĒKMnO4ÓėĘäĖūĪļÖŹ·“Ó¦)ŌŚµĪ¶Ø¹ż³ĢÖŠ,ČōĪ“ÓƱź×¼ŅŗČóĻ“µĪ¶Ø¹Ü,ŌņŹ¹²ā¶Ø½į¹ū””””””””(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±),µĪ¶ØÖÕµćµÄĻÖĻóŹĒ”” ”£µĪ¶Ø·ÖĪöŹ±,³ĘČ”a gīŃĢśæó,“¦Ąķŗó,ÓĆc mol/L KMnO4±ź×¼ŅŗµĪ¶Ø,ĻūŗÄV mL,ŌņĢśŌŖĖŲµÄÖŹĮæ·ÖŹżµÄ±ķ“ļŹ½ĪŖ”””””””””£

ŅŃÖŖ:H2TiO3ŹĒÖÖÄŃČÜÓŚĖ®µÄĪļÖŹ”£
(1)īŃĢśæóÓĆÅØĮņĖį“¦ĄķÖ®Ē°,ŠčŅŖ·ŪĖé,ĘäÄæµÄŹĒ ”””£
(2)TiO2+Ė®½āÉś³ÉH2TiO3µÄĄė×Ó·½³ĢŹ½ĪŖ ”””£
(3)¼ÓČėNaClO·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”””£
(4)ŌŚŹµŃéÖŠ,“ÓČÜŅŗÖŠ¹żĀĖ³öH2TiO3ŗó,ĖłµĆĀĖŅŗ»ė×Ē,Ó¦ČēŗĪ²Ł×÷”” ”£
(5)ĪŖ²ā¶ØīŃĢśæóÖŠĢśµÄŗ¬Įæ,ijĶ¬Ń§Č”¾ÅØĮņĖįµČ“¦ĄķµÄČÜŅŗ(“ĖŹ±īŃĢśæóÖŠµÄĢśŅŃČ«²æ×Ŗ»ÆĪŖ¶ž¼ŪĢśĄė×Ó),²ÉČ”KMnO4±ź×¼ŅŗµĪ¶ØFe2+µÄ·½·Ø:(²»æ¼ĀĒKMnO4ÓėĘäĖūĪļÖŹ·“Ó¦)ŌŚµĪ¶Ø¹ż³ĢÖŠ,ČōĪ“ÓƱź×¼ŅŗČóĻ“µĪ¶Ø¹Ü,ŌņŹ¹²ā¶Ø½į¹ū””””””””(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±),µĪ¶ØÖÕµćµÄĻÖĻóŹĒ”” ”£µĪ¶Ø·ÖĪöŹ±,³ĘČ”a gīŃĢśæó,“¦Ąķŗó,ÓĆc mol/L KMnO4±ź×¼ŅŗµĪ¶Ø,ĻūŗÄV mL,ŌņĢśŌŖĖŲµÄÖŹĮæ·ÖŹżµÄ±ķ“ļŹ½ĪŖ”””””””””£
(1)Ōö“ó¹ĢĢåµÄ±ķĆ껿,¼Óæģ»Æѧ·“Ó¦ĖŁĀŹ
(2)TiO2++2H2O H2TiO3+2H+
H2TiO3+2H+
(3)ClO-+2Fe2++2H+ 2Fe3+ +Cl- +H2O
2Fe3+ +Cl- +H2O
(4)»»ÉĻŠĀµÄ¹żĀĖĘ÷,½«»ė×ĒĀĖŅŗÖŲŠĀ¹żĀĖ
(5)Ę«øß””µĪ¼Ó×īŗóŅ»µĪKMnO4±ź×¼Ņŗ,ČÜŅŗ±ä³É×ĻŗģÉ«,ĒŅŌŚ°ė·ÖÖÓÄŚ²»ĶŹÉ«ĪŖÖ¹””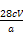 %
%
(2)TiO2++2H2O
 H2TiO3+2H+
H2TiO3+2H+(3)ClO-+2Fe2++2H+
 2Fe3+ +Cl- +H2O
2Fe3+ +Cl- +H2O(4)»»ÉĻŠĀµÄ¹żĀĖĘ÷,½«»ė×ĒĀĖŅŗÖŲŠĀ¹żĀĖ
(5)Ę«øß””µĪ¼Ó×īŗóŅ»µĪKMnO4±ź×¼Ņŗ,ČÜŅŗ±ä³É×ĻŗģÉ«,ĒŅŌŚ°ė·ÖÖÓÄŚ²»ĶŹÉ«ĪŖÖ¹””
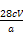 %
%(1)·ŪĖéµÄÄæµÄŹĒŌö“ó¹ĢĢåÓėČÜŅŗ·“Ó¦Ź±µÄ½Ó“„Ć껿,¼Óæģ·“Ó¦µÄ½ųŠŠ”£
(3)ĖįŠŌĢõ¼žĻĀClO-½«Fe2+Ńõ»ÆĪŖFe3+,ClO-±»»¹Ō³ÉCl-:ClO-+2Fe2++2H+ Cl-+2Fe3++H2O”£
Cl-+2Fe3++H2Oӣ
(4)ĀĖŅŗ»ė×Ē,ĖµĆ÷¹żĀĖĘ÷Ėš»µ,Ó¦»»ÉĻŠĀ¹żĀĖĘ÷ŗó,ÖŲŠĀ¹żĀĖ”£
(5)Čē¹ūĪ“ÓƱź×¼ŅŗČóĻ“µĪ¶Ø¹Ü,»įµ¼ÖĀ±ź×¼Ņŗ±»Ļ”ŹĶ,ĻūŗÄKMnO4±ź×¼ŅŗĢå»żĘ«“ó,Ź¹²ā¶Ø½į¹ūĘ«øß;µ±µĪ¶Ø“ļÖÕµćŹ±,µĪ¼Ó×īŗóŅ»µĪKMnO4±ź×¼Ņŗ,ČÜŅŗ±ä³É×ĻŗģÉ«,ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«”£ÓÉMn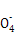 ”«5Fe2+ÖŖ,
”«5Fe2+ÖŖ,
w(Fe)=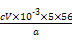 ”Į100%=
”Į100%=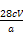 %”£
%ӣ
(3)ĖįŠŌĢõ¼žĻĀClO-½«Fe2+Ńõ»ÆĪŖFe3+,ClO-±»»¹Ō³ÉCl-:ClO-+2Fe2++2H+
 Cl-+2Fe3++H2Oӣ
Cl-+2Fe3++H2O”£(4)ĀĖŅŗ»ė×Ē,ĖµĆ÷¹żĀĖĘ÷Ėš»µ,Ó¦»»ÉĻŠĀ¹żĀĖĘ÷ŗó,ÖŲŠĀ¹żĀĖ”£
(5)Čē¹ūĪ“ÓƱź×¼ŅŗČóĻ“µĪ¶Ø¹Ü,»įµ¼ÖĀ±ź×¼Ņŗ±»Ļ”ŹĶ,ĻūŗÄKMnO4±ź×¼ŅŗĢå»żĘ«“ó,Ź¹²ā¶Ø½į¹ūĘ«øß;µ±µĪ¶Ø“ļÖÕµćŹ±,µĪ¼Ó×īŗóŅ»µĪKMnO4±ź×¼Ņŗ,ČÜŅŗ±ä³É×ĻŗģÉ«,ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«”£ÓÉMn
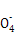 ”«5Fe2+ÖŖ,
”«5Fe2+ÖŖ,w(Fe)=
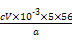 ”Į100%=
”Į100%=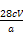 %”£
%ӣ
Į·Ļ°²įĻµĮŠ“š°ø
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
Ļą¹ŲĢāÄæ
 ĪŽ³Įµķ
ĪŽ³Įµķ °×É«³Įµķ
°×É«³Įµķ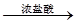 ĘųĢå
ĘųĢå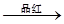 ĶŹÉ«
ĶŹÉ«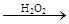 ×Ų»ĘÉ«ČÜŅŗ
×Ų»ĘÉ«ČÜŅŗ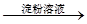 Ą¶É«ČÜŅŗ
Ą¶É«ČÜŅŗ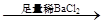 °×É«³Įµķ
°×É«³Įµķ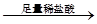 ³ĮµķČܽā
³ĮµķČܽā