��Ŀ����
����ʵ���в�������������۶�Ӧ��ϵ��ȷ��һ����(����)
| | ʵ����� | ʵ������ | ʵ����� |
| A | �����ۺ������м������������������Һ,��ȫ��Ӧ����� | ������ɫ���� | ���Գ�ȥ�����л��е��������� |
| B | ��������ͨ����ˮ�� | ��Һ��ɫ | ����������Ư���� |
| C | ��ij��Һ�м���NaOH���� | �����ܹ�ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���� | ����Һ��һ������N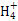 |
| D | ���ۺ�ϡ�����Ϲ��Ⱥ�,�ټ���������������ͭ����Һ | ����ש��ɫ���� | ����ˮ������������� |
C
����������������Һ(����)��Ӧ,�����������ɫ����,���۲�������������Һ��Ӧ,A����;��ˮ��ɫ��ԭ�����䱻SO2��ԭ,B����;ʹʪ��ĺ�ɫʯ����ֽ�����������ǰ���,�����ǿ��Ȳ�������,C��ȷ;ʵ������Һ�����Զ�������ש��ɫ����,D����

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ


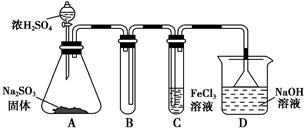
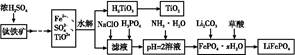
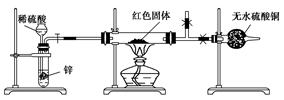
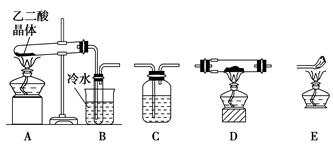
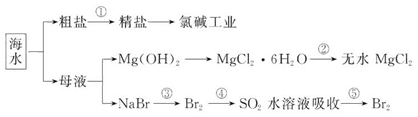
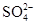 ��Ca2+��Mg2+��Fe3+�����ʣ������ҩƷ˳��Ϊ��Na2CO3��Һ��NaOH��Һ��BaCl2��Һ�����˺������
��Ca2+��Mg2+��Fe3+�����ʣ������ҩƷ˳��Ϊ��Na2CO3��Һ��NaOH��Һ��BaCl2��Һ�����˺������