��Ŀ����
���й�����Һ���������ӵļ��������ȷ���� ����������
| A�����백ˮʱ���ɰ�ɫ����������ˮ����ʱ��ɫ������ʧ����ԭ��Һ��һ����Al3������ |
| B��������FeCl2��Һ��AlCl3��Һ��AgNO3��Һ����3�ִ���Һ�зֱ�μ�������ˮ |
| C���������ᣬ����ʹ����ʯ��ˮ����ǵ��������ɣ���ԭ��Һ��һ���д�����CO32-���� |
| D������BaCl2��Һ���ɰ�ɫ�������ټ���������������ܽ⣬��ԭ��Һ��һ����SO42-���� |
B
ѡ��A�����ɵ�Al��OH��3���������ڹ����İ�ˮ��A����ѡ��C��ʵ������˵����Һ�п��ܴ���CO32-��HCO3-��SO32-�ȣ�C����ѡ��D��ʵ������˵����Һ�п��ܴ���SO42-��Ag����D����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
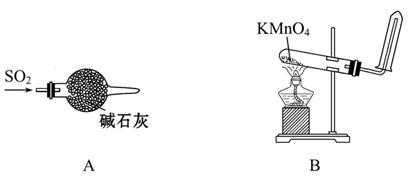
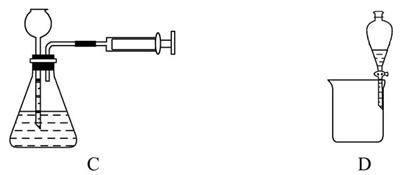
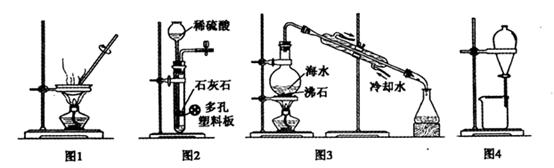
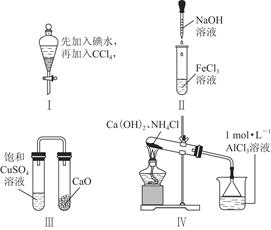
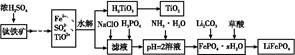
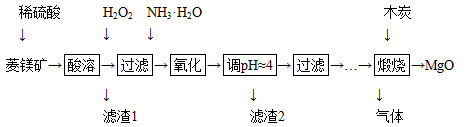
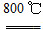 2MgO��2SO2����CO2��
2MgO��2SO2����CO2��