��Ŀ����
��֪����1 mol C��H����Ҫ��������414.4 kJ������1 mol C��C����Ҫ��������347.4 kJ������1 mol C===C������ų�����615.3 kJ������1 mol H��H������ų�����435.3 kJ���л���������һ�������·ֽ�ķ�Ӧ�ɱ�ʾΪ��
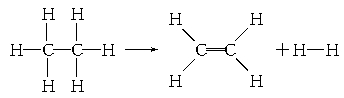
���ڷ�Ӧ��������1 mol���飬���йظ÷�Ӧ��˵����ȷ����

���ڷ�Ӧ��������1 mol���飬���йظ÷�Ӧ��˵����ȷ����
| A���÷�Ӧ�ų�251.2 kJ������ | B���÷�Ӧ����251.2 kJ������ |
| C���÷�Ӧ�ų�125.6 kJ������ | D���÷�Ӧ����125.6 kJ������ |
D
�����������Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ������ݷ���ʽ�ͼ��ܿ�֪���÷�Ӧ�ķ�Ӧ�ȣ�414.4kJ/mol��6��347.4 kJ/mol��414.4kJ/mol��4��615.3kJ/mol��435.3kJ/mol����125.2kJ/mol����˸÷�Ӧ�����ȷ�Ӧ����ѡD��
�����������Ǹ߿��еij������ͣ����ڻ���������Ŀ��顣����Ĺؼ�����ȷ��Ӧ�Ⱥͼ��ܵĹ�ϵ���Լ���������ݣ�����������ѧ���������������ͷ�ɢ˼ά������

��ϰ��ϵ�д�
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
�����Ŀ

 CH3OH(g) ��H��QkJ/mol
CH3OH(g) ��H��QkJ/mol ( CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ������ͼ2��ʾ����������Ӧ��Q_____0���������������������
( CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ������ͼ2��ʾ����������Ӧ��Q_____0��������������������� CH3CH2OH(g) + H2O(g) ��H =" ��256.1" kJ��mol��1
CH3CH2OH(g) + H2O(g) ��H =" ��256.1" kJ��mol��1


 O2(g)��ZnO(s) ��H ����351.1kJ��mol��1
O2(g)��ZnO(s) ��H ����351.1kJ��mol��1

 2NH3�������仯��ͼ��ʾ��
2NH3�������仯��ͼ��ʾ��
 N2(g)+
N2(g)+ H2(g)
H2(g)