��Ŀ����
��У��ѧ��ȤС���ѧ����ijƷ�Ƶ�����Һ�ijɷֺ����ʽ���ʵ��̽����
�ٸ�����Һ�����ɫ����ȡ�����μ�AgNO3��Һ���ɰ�ɫ�������ó������������ᣩ��
���ø���ྻ������պȡ����Һ���㵽pH��ֽ�ϣ���ֽ�ȱ�������ɫ��
��ȡ��������Һ���μ�ϡ������л���ɫ�������ɣ�
���ýྻ��˿պȡ����Һ������ɫ���������գ�����ʻ�ɫ��
��ȡ��������Һ��ͨ������H2S���壬�ȿ����С�dz��ɫ�����������֡����塱��ȡ������Һ�������μ�BaCl2��Һ���а�ɫ�������ɣ��ó������������ᣩ��
��ش��������⣺
��1��������Һ����Ҫ�ɷ���_______________________________________��
��2��pH��ֽ��ɫ�ı仯˵������Һ��Һ���е������� _________________��
��3��ʵ����е����ӷ���ʽΪ_______________________________________��
��4��ʵ����У��С�dz��ɫ����������ʱ�����ӷ���ʽΪ_________________________���֡����塱ʱ�����ӷ���ʽΪ__________________________________________��
��1��NaCl��NaClO��
��2�����ԣ�Ư���ԣ�
��3��Cl-+ClO-+2H+=Cl2��+H2O
��4��H2S+ClO-=S��+Cl-+H2O��S+3ClO-+2OH-=SO42-+3Cl-+H2O����S+3ClO-+H2O=SO42-+3Cl-+2H+��
�������������������Һ�����ɫ����ȡ�����μ�AgNO3��Һ���ɰ�ɫ�������ó������������ᣩ��֤������Cl-���ø���ྻ������պȡ����Һ���㵽pH��ֽ�ϣ���ֽ�ȱ�������ɫ,֤����Һ�ʼ��ԡ��������ԣ�����Һ�μ�ϡ������л���ɫ�������ɣ�֤���������Ե�����ClO-��������Һ��ɫ��Ӧ�ʻ�ɫ��˵����Na+���������Һ��ͨ������H2S���壬�ȿ����С�dz��ɫ�����������֡����塱��ȡ������Һ�������μ�BaCl2��Һ���а�ɫ�������ɣ��ó������������ᣩ��˵�������ɲ�����ˮ��S���ʣ�Ȼ��S�����ֱ�������SO42-���õ��Ȳ�����ˮҲ���������BaSO4������
���㣺�������ӵļ��������ӹ��桢�����Ӳμӵ�������ԭ��Ӧ��֪ʶ��

 ��У����ϵ�д�
��У����ϵ�д��о���������������ȴ�����Ⱦ�������������Ҫ���塣
��1��úȼ�ղ����������к��У�SO2��CO2��PM2.5�ȣ���������ֱ���ŷŵ������У���������Ҫ���������� ������д��ĸ��ţ�
| A������ЧӦ | B������ | C���۳���Ⱦ | D��ˮ�帻Ӫ���� |
������̼��ʯ��ʯ��Һ��Ӧ�õ��IJ���Ϊ ��
��������Ʊ���������������������ƵĻ�ѧ����ʽΪ ��
��2�����������һ�������ȼҵ��Ʒ������������������ķ����������������£�
 ��ҵ�����У�����۵ķ�Ӧ����Ϊ ��
��ҵ�����У�����۵ķ�Ӧ����Ϊ ���ù���������D������Ϊԭ��ѭ�����ã����Ļ�ѧʽΪ ��
д������ڷ�Ӧ�����ӷ���ʽ ��
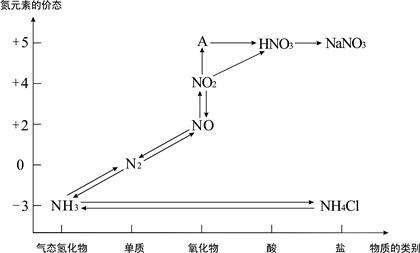
��3������β���к��еĵ������NOx�����γ����꣬д��NO2ת��ΪHNO3�Ļ�ѧ����ʽ ����β���п�������ʱ��NOX�ڴ�ת�����б���ԭ��N2�ų���д��NO��CO��ԭ�Ļ�ѧ����ʽ_ ��
��4����ҵ�ϳ���Na2CO3��Һ�������������
��֪��NO������Na2CO3��Һ��Ӧ
NO + NO2 + Na2CO3 = 2NaNO2 + CO2
2NO2 + Na2CO3 = NaNO2 + NaNO3 + CO2
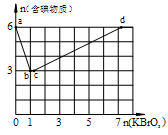
����������Na2CO3��Һ��ȫ����NO��NO2�Ļ������8.96L�����������NO��NO2�������Ϊ1��3�������չ����в��������ڱ�״���µ����Ϊ ��

 2NO
2NO CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O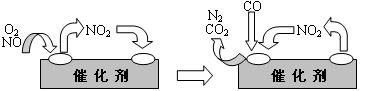
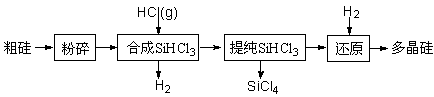
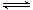 Si (s) + 3HCl (g) ��H ��0����ƽ�ⳣ������ʽΪK = ��Ϊ���ԭʱSiHCl3��ת���ʣ��ɲ�ȡ�Ĵ�ʩ�� ��
Si (s) + 3HCl (g) ��H ��0����ƽ�ⳣ������ʽΪK = ��Ϊ���ԭʱSiHCl3��ת���ʣ��ɲ�ȡ�Ĵ�ʩ�� ��

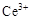 ��
�� ������Ҫ������ʽ��
������Ҫ������ʽ��

 R-CH��OH��SO3Na
R-CH��OH��SO3Na