��Ŀ����
�о���������������ȴ�����Ⱦ�������������Ҫ���塣
��1��úȼ�ղ����������к��У�SO2��CO2��PM2.5�ȣ���������ֱ���ŷŵ������У���������Ҫ���������� ������д��ĸ��ţ�
| A������ЧӦ | B������ | C���۳���Ⱦ | D��ˮ�帻Ӫ���� |
������̼��ʯ��ʯ��Һ��Ӧ�õ��IJ���Ϊ ��
��������Ʊ���������������������ƵĻ�ѧ����ʽΪ ��
��2�����������һ�������ȼҵ��Ʒ������������������ķ����������������£�
 ��ҵ�����У�����۵ķ�Ӧ����Ϊ ��
��ҵ�����У�����۵ķ�Ӧ����Ϊ ���ù���������D������Ϊԭ��ѭ�����ã����Ļ�ѧʽΪ ��
д������ڷ�Ӧ�����ӷ���ʽ ��
��3������β���к��еĵ������NOx�����γ����꣬д��NO2ת��ΪHNO3�Ļ�ѧ����ʽ ����β���п�������ʱ��NOX�ڴ�ת�����б���ԭ��N2�ų���д��NO��CO��ԭ�Ļ�ѧ����ʽ_ ��
��4����ҵ�ϳ���Na2CO3��Һ�������������
��֪��NO������Na2CO3��Һ��Ӧ
NO + NO2 + Na2CO3 = 2NaNO2 + CO2
2NO2 + Na2CO3 = NaNO2 + NaNO3 + CO2
����������Na2CO3��Һ��ȫ����NO��NO2�Ļ������8.96L�����������NO��NO2�������Ϊ1��3�������չ����в��������ڱ�״���µ����Ϊ ��
��1��ABC(2�� ©ѡ��1��)
Ca(HCO3)2��̼�����
Ca(HSO3)2+O2=CaSO4+H2SO4
��2����ȼ NaCl SO2+OH��= HSO3��
��3��3NO2+H2O=2HNO3+NO
2NO+2CO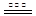 N2+2CO2
N2+2CO2
��4��4.48L��4��)
���������������1��SO2�������� ��CO2����������ЧӦ��PM2.5�۳���Ⱦ�ȹʴ�ѡ��ABC���������ķ���ʽΪCa(HSO3)2+O2=CaSO4+H2SO4 ��ҵ��������ķ�Ӧ����Ϊ��ȼ�����������ƺ����ᷴӦ�������Ȼ��ƺͶ���������ڷ�Ӧ�����ӷ���ʽSO2+OH��= HSO3����
��3��NO2ת��ΪHNO3�Ļ�ѧ����ʽ3NO2+H2O=2HNO3+NO����������Ϣ��д��NO��CO��ԭ�Ļ�ѧ����ʽ2NO+2CO N2+2CO2����4��NO��NO2�Ļ������8.96L���ʵ���Ϊ0.4mol���ֻ��������NO��NO2�������Ϊ1��3����NO��NO2�ֱ�Ϊ0.1mol��0.3mol��������֪����ʽNO + NO2 + Na2CO3 = 2NaNO2 + CO2��NO2��ʣ��0.2mol�������˵ڶ�����Ӧ 2NO2 + Na2CO3 = NaNO2 + NaNO3 + CO2�����˶�����̼0.1mol�������չ����в��������ڱ�״���µ����Ϊ4.48L��
N2+2CO2����4��NO��NO2�Ļ������8.96L���ʵ���Ϊ0.4mol���ֻ��������NO��NO2�������Ϊ1��3����NO��NO2�ֱ�Ϊ0.1mol��0.3mol��������֪����ʽNO + NO2 + Na2CO3 = 2NaNO2 + CO2��NO2��ʣ��0.2mol�������˵ڶ�����Ӧ 2NO2 + Na2CO3 = NaNO2 + NaNO3 + CO2�����˶�����̼0.1mol�������չ����в��������ڱ�״���µ����Ϊ4.48L��
���㣺���ո�������ͼ���Ʋ����ʵĻ�ѧʽ����������֪��Ϣ���л�ѧ���㡣

 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�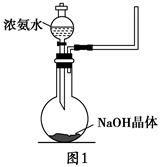
 NH4+��OH����NaOH����ʹ�ÿ��淴Ӧ��ƽ�������ƶ�
NH4+��OH����NaOH����ʹ�ÿ��淴Ӧ��ƽ�������ƶ�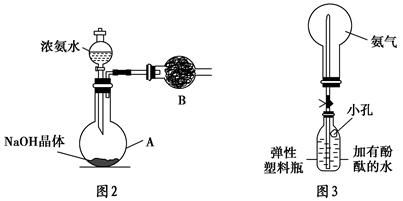
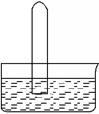


 2Cl2��2H2O����ʵ���ȵ�ѭ�����á�
2Cl2��2H2O����ʵ���ȵ�ѭ�����á�