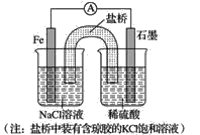
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ±ΨΧβ…φΦΑ≤ΩΖ÷Ά≠ΦΑΤδΜ·ΚœΈοΒΡœύΙΊΫαΙΙΈ ΧβΒΡΩΦ≤ιΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΓΘ
(1)–¥≥ωΜυΧ§Cu2+ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΘΚ________ΓΘCuΒΡΆ§÷ήΤΎ‘ΣΥΊ÷–Θ§”κΆ≠‘≠Ή”ΉνΆβ≤ψΒγΉ” ΐœύΒ»ΒΡ‘ΣΥΊ‘≠Ή”ΜΙ”–___________(”Ο‘ΣΥΊΖϊΚ≈±μ Ψ)ΓΘ
(2)¥”‘≠Ή”ΫαΙΙΫ«Ε»Ζ÷ΈωΗΏΈ¬Cu2O±»CuOΈ»Ε®ΒΡ‘≠“ρ «___________ΓΘ
(3)[Cu(NH3)2]AcΩ…”Ο”ΎΚœ≥…Α±ΙΛ“Β÷–ΒΡΆ≠œ¥ΙΛ–ρΘ§≥ΐ»ΞΫχ»ΥΚœ≥…Υΰ«ΑΜλΚœΤχ÷–ΒΡCO(COΡή Ι¥ΏΜ·ΦΝ÷–ΕΨ)ΓΘ
ΔΌAc±μ ΨCH3COO-Θ§Ac÷–ΧΦ‘≠Ή”ΒΡ‘”Μ·ΖΫ ΫΈΣ________ΓΘ
ΔΎ[Cu(NH3)2]AcΡήΙΜΫαΚœCOΒΡ‘≠“ρ «________ΓΘ
(4)Ά≠‘≠Ή””ꬻ‘≠Ή”–Έ≥…Μ·ΚœΈοΒΡΨßΑϊ»γΆΦΥυ Ψ(ΚΎ«ρ¥ζ±μΆ≠‘≠Ή”Θ§ΑΉ«ρ±μ Ψ¬»‘≠Ή”)ΓΘ
ΔΌ“―÷ΣΆ≠ΚΆ¬»ΒΡΒγΗΚ–‘Ζ÷±πΈΣ1.9ΚΆ3.0Θ§‘ρΆ≠”ꬻ–Έ≥…ΒΡΜ·ΚœΈο τ”Ύ________Χν(ΓΑάκΉ”Γ±ΜρΓΑΙ≤ΦέΓ±)Μ·ΚœΈοΓΘ
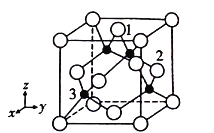
ΔΎ“‘ΨßΑϊ≤Έ ΐΈΣΒΞΈΜ≥ΛΕ»Ϋ®ΝΔΒΡΉχ±ξœΒΩ…“‘±μ ΨΨßΑϊ÷–Ης‘≠Ή”ΒΡΈΜ÷ΟΘ§≥ΤΉς‘≠Ή”Ζ÷ ΐΉχ±ξΘ§άΐ»γΆΦ÷–‘≠Ή”1ΒΡΉχ±ξΈΣ(![]() Θ§
Θ§![]() Θ§1)Θ§‘ρ‘≠Ή”2ΚΆ3ΒΡΉχ±ξΖ÷±πΈΣ___________ΓΔ__________ΓΘ
Θ§1)Θ§‘ρ‘≠Ή”2ΚΆ3ΒΡΉχ±ξΖ÷±πΈΣ___________ΓΔ__________ΓΘ
Δέ“―÷ΣΗΟΨßΧεΒΡΟήΕ»ΈΣΠ―g/cm3Θ§ΑΔΖϋΦ”Β¬¬ό≥Θ ΐΈΣNAΘ§‘ρΗΟΨßΧε÷–Ά≠‘≠Ή”ΚΆ¬»‘≠Ή”ΒΡΉνΕΧΨύάκΈΣ_____________pm(÷Μ–¥ΦΤΥψ Ϋ)ΓΘ
ΓΨ¥πΑΗΓΩ[Ar]3d9 KΓΔCr Cu+ΒΡΦέ≤ψΒγΉ”≈≈≤ΦΈΣ3d10Θ§3dΙλΒά»Ϊ≥δ¬ζΘ§ΫœΈ»Ε® sp2ΚΆsp3 –Έ≥…ΝΥ≈δΈΜΦϋ Ι≤Φέ (![]() Θ§1Θ§
Θ§1Θ§![]() ) (
) (![]() Θ§
Θ§![]() Θ§
Θ§![]() )
) 
ΓΨΫβΈωΓΩ
(1)CuΈΣ29Κ≈‘ΣΥΊΘ§‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ[Ar]3d104s1Θ§Ι Cu2+ΒΡΚΥΆβΒγΉ”≈≈≤ΦΈΣ[Ar]3d9Θ§CuΉνΆβ≤ψΒγΉ” ΐΈΣ1ΗωΘ§”κΆ≠ΉνΆβ≤ψΒγΉ” ΐœύΆ§ΒΡΜΙ”–KΚΆCrΘ§CrΒΡΚΥΆβΒγΉ”≈≈≤ΦΈΣ[Ar]3d54s1ΓΘ
(2)”…”ΎCu2O÷–Cu+Φέ≤ψΒγΉ”≈≈≤ΦΈΣ3d10Θ§3dΙλΒά»Ϊ≥δ¬ζΘ§ΫœΈ»Ε®Θ§Υυ“‘ΗΏΈ¬Cu2O±»CuOΈ»Ε®ΓΘ
(3)ΔΌCH3COO-÷–ΦΉΜυ…œΒΡCΈόΙ¬ΒγΉ”Ε‘Θ§–Έ≥…4ΧθΦϋΘ§ τ”Ύsp3‘”Μ·Θ§θΞΜυ…œΒΡΧΦ‘≠Ή”ΈόΙ¬ΒγΉ”Ε‘Θ§–Έ≥…3ΧθΦϋΘ§ΈΣsp2‘”Μ·ΓΘ
ΔΎ”…”ΎCOΒΡCΧαΙ©Ι¬Ε‘ΒγΉ””κCu+–Έ≥…ΝΥ≈δΈΜΦϋΘ§Υυ“‘[Cu(NH3)2]AcΡήΙΜΫαΚœCOΓΘ
(4)ΔΌ“ΜΑψ»œΈΣΒγΗΚ–‘œύ≤ν–Γ”Ύ1.7–Έ≥…Ι≤ΦέΦϋΘ§“―÷ΣΆ≠ΚΆ¬»ΒΡΒγΗΚ–‘Ζ÷±πΈΣ1.9ΚΆ3.0Θ§‘ρΆ≠”ꬻ–Έ≥…ΒΡΜ·ΚœΈο τ”ΎΙ≤ΦέΜ·ΚœΈοΓΘ
ΔΎΗυΨί‘≠Ή”ΒΡΉχ±ξΚΆΉχ±ξœΒΘ§άύ±»Ω…ΒΟ‘≠Ή”2ΒΡΉχ±ξΈΣ(![]() Θ§1Θ§
Θ§1Θ§![]() )Θ§‘≠Ή”3ΒΡΉχ±ξΈΣ(
)Θ§‘≠Ή”3ΒΡΉχ±ξΈΣ(![]() Θ§
Θ§![]() Θ§
Θ§![]() )ΓΘ
)ΓΘ
ΔέCuΚΆClΒΡΉνΕΧΨύάκΦ¥ΈΣΨßΑϊΧεΕ‘Ϋ«œΏΒΡ![]() Θ§…ηΨßΑϊ≤Έ ΐΈΣa cmΘ§‘ρ
Θ§…ηΨßΑϊ≤Έ ΐΈΣa cmΘ§‘ρ![]() Θ§
Θ§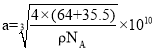 pmΘ§ΉνΕΧΨύάκΈΣ
pmΘ§ΉνΕΧΨύάκΈΣ![]() Θ§Φ¥
Θ§Φ¥ pmΓΘ
pmΓΘ

 Οϊ≈Τ÷–―ßΩΈ ±Ής“ΒœΒΝ–¥πΑΗ
Οϊ≈Τ÷–―ßΩΈ ±Ής“ΒœΒΝ–¥πΑΗ ΟςΧλΫΧ”ΐΩΈ ±ΧΊ―ΒœΒΝ–¥πΑΗ
ΟςΧλΫΧ”ΐΩΈ ±ΧΊ―ΒœΒΝ–¥πΑΗ ’ψΫ≠–¬ΩΈ≥Χ»ΐΈ§ΡΩ±ξ≤βΤάΩΈ ±ΧΊ―ΒœΒΝ–¥πΑΗ
’ψΫ≠–¬ΩΈ≥Χ»ΐΈ§ΡΩ±ξ≤βΤάΩΈ ±ΧΊ―ΒœΒΝ–¥πΑΗ ÷ή÷ή«εΦλ≤βœΒΝ–¥πΑΗ
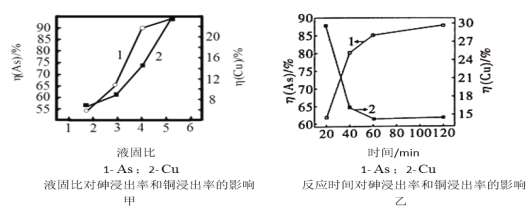
÷ή÷ή«εΦλ≤βœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ÷–Ρœ¥σ―ß÷Θ―≈ΫήΒ»3ΈΜάœ ΠΧα≥ωΓΑ“‘Κ§…ιΖœΥ°≥ΝΒμΜΙ‘≠Ζ®÷Τ±ΗAs2O3Γ±Θ§”–ΫœΗΏΒΡ ΒΦ ”Π”ΟΦέ÷ΒΓΘΡ≥ΙΛ≥ßΚ§…ιΖœΥ°Κ§”–H3AsO3ΓΔH2SO4ΓΔFe2(SO4)3ΓΔBi2(SO4)3Β»Θ§άϊ”ΟΗΟΖœΥ°Χα»ΓAs2O3ΒΡΝς≥Χ»γΆΦΥυ ΨΓΘ

±μΘΚΫπ τάκΉ”≥ΝΒμpH÷Β±μΗώ(20Γφ)
10-1 | 10-2 | 10-3 | 10-4 | 10-5 | |
Fe3+ | 1.8 | 2.2 | 2.5 | 2.9 | 3.2 |
Cu2+ | 4.7 | 5.2 | 5.7 | 6.2 | 6.7 |
(1)ΈΣΝΥΦ”Ωλ÷–ΚΆΙΐ≥ΧΒΡΥΌ¬ Θ§Ω…“‘≤…»ΓΒΡ¥κ ©”–______________(–¥≥ω“ΜΧθΚœάμΒΡ¥κ ©Φ¥Ω…)ΓΘ
(2)≥ΝΒμ÷–ΒΡ≥…Ζ÷Θ§≥ΐΝΥBi(OH)3≥ΝΒμΆβΘ§ΜΙ”–_________ΓΘ
(3)AΩ…“‘―≠ΜΖάϊ”ΟΘ§AΒΡΜ·―ß ΫΈΣ_________ΓΘ‘Ύ¬Υ“Κ1÷–Θ§Φ”»κNaOHΒςΫΎpHΈΣ8ΒΡΡΩΒΡ «_______ΓΘ
(4)Cu3(AsO3)2≥ΝΒμΦ”»κ“ΜΕ®ΝΩΒΡΥ°Βς≥…Ϋ§ΝœΘ§Ά®»κSO2Θ§ΗΟΙΐ≥ΧΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ Ϋ «__________ΓΘ
(5)Α¥’’“ΜΕ®ΒΡ“ΚΙΧ±»Θ§ΫΪΥ°Φ”»κCu3(AsO3)2≥ΝΒμ÷–Θ§Βς≥…Ϋ§ΝœΓΘΒ±Ζ¥”ΠΈ¬Ε»ΈΣ25ΓφΘ§SO2ΝςΝΩΈΣ16L/hΘ§“ΚΙΧ±»ΓΔ ±ΦδΕ‘…ιΓΔΆ≠Ϋΰ≥ω¬ ΒΡ”Αœλ»γΆΦΦΉΓΔ““Υυ ΨΓΘ«κ―Γ‘ώΉν “ΥΒΡ“ΚΙΧ±»ΓΔΖ¥”Π ±ΦδΘΚ______________ΓΔ_______________ΓΘ
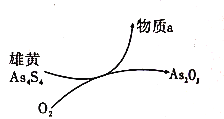
(6)“ΜΕ®ΧθΦΰœ¬Θ§”Ο–έΜΤ(As4S4)÷Τ±ΗAs2O3ΒΡΉΣΜ·ΙΊœΒ»γΆΦΥυ ΨΓΘ»τΖ¥”Π÷–Θ§1mol As4S4(Τδ÷–As‘ΣΥΊΒΡΜ·ΚœΦέΈΣ+2Φέ)≤ΈΦ”Ζ¥”Π ±Θ§ΉΣ“Τ28mole-Θ§‘ρΈο÷ aΈΣ_______(ΧνΜ·―ß Ϋ)ΓΘ
ΓΨΧβΡΩΓΩ Β―ι “÷Τ±Η εΕΓΜυ±Ϋ(![]() )ΒΡΖ¥”ΠΚΆ”–ΙΊ ΐΨί»γœ¬ΘΚ
)ΒΡΖ¥”ΠΚΆ”–ΙΊ ΐΨί»γœ¬ΘΚ
![]() +ClC(CH3)3
+ClC(CH3)3![]()
![]() +HCl
+HCl
Έο÷ | œύΕ‘Ζ÷Ή”÷ ΝΩ | ΟήΕ»/gcm-3 | »έΒψ/Γφ | Ζ–Βψ/Γφ | »ήΫβ–‘ |
AlCl3 | ΓΣΓΣ | ΓΣΓΣ | 190 | “Ή…ΐΜΣ | ”ωΥ°ΦΪ“Ή≥±Ϋβ≤Δ≤ζ…ζΑΉ…Ϊ―ΧΈμΘ§ΈΔ»ή”Ύ±Ϋ |
±Ϋ | 78 | 0.88 | ΓΣΓΣ | 80.1 | Ρ―»ή”ΎΥ°Θ§“Ή»ή”Ύ““¥Φ |
¬»¥ζ εΕΓΆι | 92.5 | 1.85 | ΓΣΓΣ | 51.6 | Ρ―»ή”ΎΥ°Θ§Ω…»ή”Ύ±Ϋ |
εΕΓΜυ±Ϋ | 134 | 0.87 | ΓΣΓΣ | 169 | Ρ―»ή”ΎΥ°Θ§“Ή»ή”Ύ±Ϋ |
I.»γΆΦ « Β―ι “÷Τ±ΗΈόΥ°AlCl3Ω…Ρή–η“ΣΒΡΉΑ÷ΟΘΚ
A.  B.
B.  C.
C.  D.
D.  E.
E.  F.
F. 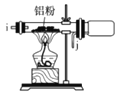
(1)Φλ≤ιBΉΑ÷ΟΤχΟή–‘ΒΡΖΫΖ® «__________ΓΘ
(2)÷Τ±ΗΈόΥ°AlCl3ΒΡΉΑ÷ΟΒΡΚœάμΒΡΝ§Ϋ”Υ≥–ρΈΣ________(Χν–Γ–¥Ή÷ΡΗ)Θ§Τδ÷–EΉΑ÷ΟΒΡΉς”Ο «___________ΓΘ
(3) Β―ι ±”Πœ»Φ”»»‘≤ΒΉ…’ΤΩ‘ΌΦ”»»”≤÷ ≤ΘΝßΙήΘ§Τδ‘≠“ρ «________ΓΘ
II.»γΆΦ « Β―ι “÷Τ±Η εΕΓΜυ±ΫΒΡΉΑ÷Ο(Φ–≥÷ΉΑ÷Ο¬‘)ΘΚ

‘Ύ»ΐΨ±…’ΤΩ÷–Φ”»κ50mLΒΡ±ΫΚΆ ΝΩΒΡΈόΥ°AlClΘ§”…Κψ―Ι¬©ΕΖΒΈΦ”¬»¥ζ εΕΓΆι10 mLΘ§“ΜΕ®Έ¬Ε»œ¬Ζ¥”Π“ΜΕΈ ±ΦδΚσΘ§ΫΪΖ¥”ΠΚσΒΡΜλΚœΈοœ¥Β”Ζ÷άκΘ§‘ΎΥυΒΟ≤ζΈο÷–Φ”»κ…ΌΝΩΈόΥ°MgSO4ΙΧΧεΘ§Ψ≤÷ΟΘ§Ιΐ¬ΥΘ§’τΝσΒΟ εΕΓΜυ±Ϋ20 gΓΘ
Ι”ΟΚψ―Ι¬©ΕΖΒΡ”≈Βψ «_________ΘΜΦ”»κΈόΥ°MgSO4ΙΧΧεΒΡΉς”Ο «____________________________________ΓΘ
(5)œ¥Β”ΜλΚœΈο ±Υυ”ΟΒΡ ‘ΦΝ”–»γœ¬»ΐ÷÷Θ§’ΐ»ΖΒΡ Ι”ΟΥ≥–ρ «__________(Χν–ρΚ≈)
ΔΌ5%ΒΡNa2CO3»ή“Κ ΔΎœΓ―ΈΥα ΔέH2O
(6)±Ψ Β―ι÷– εΕΓΜυ±ΫΒΡ≤ζ¬ ΈΣ______________(±ΘΝτ3ΈΜ”––ß ΐΉ÷)