��Ŀ����
����Ŀ������ȲΪԭ��,ͨ����ͼ��ʾ�����ܺϳ�һ�ָ߷��ӵ���G(ת�������еIJ��ַ�Ӧ���������ֲ�������ȥ)��
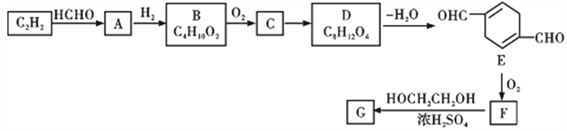
����A��B��C��D�ֱ����һ���л���,B����������
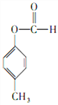
��֪��-C![]() CH+
CH+

��ش��������⣺
��1��A�к�������������Ϊ________������A�ķ�Ӧ������__________��B�Ļ�ѧ����Ϊ__________________��
��2��B��C�Ļ�ѧ��Ӧ����ʽ____________________��F��H2��ȫ�ӳɵõ������ʵĽṹ��ʽ��_________��
��3��д����������������E��ͬ���칹��Ľṹ��ʽ_______________��
�ٺ��б��� ���������� �ۿɷ���������Ӧ �ܱ����������ֲ�ͬ��������ԭ��
��4������˵����ȷ����_______(����ĸ����)
a�����������У���Ȳ���л���B���л���E���ɷ����ۺϷ�Ӧ
b���л���E������ԭ�ӹ�ƽ��
c��35%~40%�ļ�ȩ(HCHO)��Һ�׳Ƹ���������Һ����ʹ�����ʱ���
��5����д��F����G�Ļ�ѧ����ʽ________________________��
��6�����������Ϣ,д���ü�ȩ���Ҵ�Ϊԭ���Ʊ������Ĵ�[C(CH2OH)4]�ĺϳ�·��(�����Լ���ѡ)________��
���𰸡� �ǻ� �ӳɷ�Ӧ 1,4-������ HOCH2CH2CH2CH2OH+O2 ![]() OHCCH2CH2CHO+2H2O
OHCCH2CH2CHO+2H2O ![]()
 ac
ac 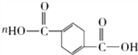 +nHOCH2CH2
+nHOCH2CH2![]()
 +(2n-l)H2O CH3CH2OH
+(2n-l)H2O CH3CH2OH![]() CH3CHO
CH3CHO![]() (CH2OH)3CCHO
(CH2OH)3CCHO![]() C(CH2OH)4
C(CH2OH)4
������������ת����ϵ�������Ϣ1������֪A�Ľṹ��ʽΪHOCH2CCCH2OH��B�Ľṹ��ʽΪHOCH2CH2CH2CH2OH��C�Ľṹ��ʽΪOHCCH2CH2CHO����Ӧ����Ϣ2��D�Ľṹ��ʽΪ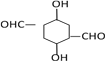 ����F�Ľṹ��ʽΪ
����F�Ľṹ��ʽΪ![]() ��
��
(1)������������֪��A�к��������ŵ�����Ϊ�ǻ���A����ȩ���е�C=O˫���ϼӳɶ����ɵģ����Է�Ӧ����Ϊ�ӳɷ�Ӧ��B�Ļ�ѧ����Ϊ1,4-��������
(2) B��C�Ļ�ѧ��Ӧ����ʽΪHOCH2CH2CH2CH2OH+O2![]() OHCCH2CH2CHO+2H2O��F��H2��ȫ�ӳɵõ������ʵĽṹ��ʽ��
OHCCH2CH2CHO+2H2O��F��H2��ȫ�ӳɵõ������ʵĽṹ��ʽ��![]() ��
��
(3)��֪E�Ľṹ��ʽΪ![]() �������������ܷ���������Ӧ����Ϊ�������������������ֲ�ͬ�������⣬������������λȡ��������һ���Ǽ���������һ���Ǽ������Է���������E��ͬ���칹��Ľṹ��ʽΪ
�������������ܷ���������Ӧ����Ϊ�������������������ֲ�ͬ�������⣬������������λȡ��������һ���Ǽ���������һ���Ǽ������Է���������E��ͬ���칹��Ľṹ��ʽΪ![]() ��
��
(4)a����Ȳ�������������Ӿ۷�Ӧ���л���B�к��С�OH�����뺬�С�COOH�����ʷ������۷�Ӧ���л���E�к��С�CHO������������ʷ������۷�Ӧ������a��ȷ��b���л���E�к�������������ṹ�ġ�CH2��ԭ���ţ�������ȩ������Ԫ��Ҳ��һ�����棬����b����c��35%~40%�ļ�ȩ(HCHO)��Һ�׳Ƹ���������Һ����ʹ�����ʱ��ԣ���c��ȷ����˱�����ȷ��Ϊac��
(5) n![]() +nHOCH2CH2OH
+nHOCH2CH2OH![]()
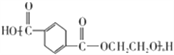 +(2n-lspan>)H2O
+(2n-lspan>)H2O
(6)�Ҵ�����������ȩ����ȩ����3������H������3���ӵļ�ȩ�����ӳɷ�Ӧ��Ȼ���ټ��ԭ���ò��ת����ϵΪ��CH3CH2OH![]() CH3CHO
CH3CHO![]() (CH2OH)3CCHO
(CH2OH)3CCHO![]() C(CH2OH)4��
C(CH2OH)4��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ������ʵ���ܴﵽԤ��Ŀ�ĵ���
��� | ʵ������ | ʵ��Ŀ�� |
A | ��FeCl3������������Ũ�����У��ټ�����ˮϡ��������Ũ�� | �����Ȼ�����Һ |
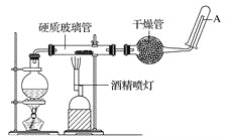
B | �����£����ɵ�ص��¶����ߵ�40����������������� | ֤���ɵ�ؿɽ���������ת��Ϊ���� |
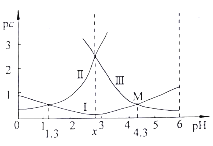
C | ��CuCl2��Һ�м���NaOH����pH>4 | ��ȥCuCl2��Fe3+���� |
D | ��BaSO4����Һ�м���Na2CO3������Һ�����˳��ij��������������������� | ֤��Ksp(BaCO3)<Ksp(BaSO4) |
A. A B. B C. C D. D