��Ŀ����
����Ŀ���ڳ����£�����ˮ������Ӧ�����ڸ����£�����ˮ�����ɷ�����Ӧ�� Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ�����������ˮ�����ķ�Ӧʵ������
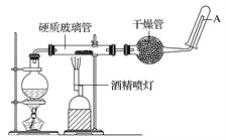
��ش��ʵ���е����⡣
��1��д���÷�Ӧ�ķ�Ӧ����ʽ��__________________________________����ָ����������ԭ��Ӧ�Ļ�ԭ����____________����������______________��
��2��ʵ��ǰ���������װ�ý��������Լ�飬����������_______________________________��
��3��Բ����ƿ��ʢװ��ˮ����װ�����Ⱥ����Ҫ������____________________________����ƿ�ײ������˼�Ƭ���Ƭ�����Ƭ��������__________________��
��4���ƾ��ƺ;ƾ���Ƶ�ȼ��˳����__________________________________��Ϊʲô��___________________________________________________________________��
��5���������ʢװ��������________________________��������_______________________��
��6���Թ����ռ���������__________�����Ҫ��A�������ܴ���ȼ�����壬�����Ը��������____________________��������________________________________����һ������Ŀ����________________________________��
���𰸡���15�֣���1��3Fe��4H2O(g)![]() Fe3O4+4H2��Fe��H2O��
Fe3O4+4H2��Fe��H2O��
��2����A�����ܲ���ˮ�У���������ƿ�����ܿ������ݣ���ȴ���γ�һ��ˮ������˵�������Ժá�
��3������ˮ��������ֹ���У����۵����壬�������ۺ�ˮ�����ĽӴ��棻
��4���ȵ�ȼ�ƾ��ƣ���ֹ����������Ӧ��
��5����ʯ�ң����������е�ˮ������
��6��H2���鴿��ȡ���Թܣ��ô�Ĵָ��ס�ܿڣ��ƽ��ƾ��ƵĻ��棬�ɿ�Ĵָ��������һ������ı����������ռ���������������������������һ������˵��������������ֹ��ȼʱ��������������ը��
��������
���⣨1������ˮ�����ڸ����·�����Ӧ������������������������Ӧ�Ļ�ѧ����ʽ�ǣ�3Fe��4H2O(g)![]() Fe3O4+4H2���ڸ÷�Ӧ�У���ʧȥ���ӣ�������������ԭ����ˮ�е���Ԫ�ػ�õ��ӣ�����ԭ��������������2��ʵ��ǰ���������װ�ý��������Լ�飬���������ǽ�A�����ܲ���ˮ�У���������ƿ�����ܿ������ݣ���ȴ���γ�һ��ˮ������˵�������Ժã���3��Բ����ƿ��ʢװ��ˮ����װ�����Ⱥ����Ҫ�����Dz���ˮ������Ϊ����ˮ������Ӧ�ṩ��Ӧ���ƿ�ײ������˼�Ƭ���Ƭ�����Ƭ�������Ƿ�ֹ���У���4���ƾ��ƺ;ƾ���Ƶ�ȼ��˳�����ȵ�ȼ�ƾ��ƣ���װ���г���ˮ�������ٵ�ȼ�ƾ���ƣ�Ŀ���Ƿ�ֹ����������Ӧ����5���������ʢװ�ǵ������Ǽ�ʯ�ң����������������е�ˮ��������6���Թ����ռ����������������������ǿ�ȼ�Ե����壬����ڵ�ȼ֮ǰ��������鴿������������ȡ���Թܣ��ô�Ĵָ��ס�ܿڣ��ƽ��ƾ��ƵĻ��棬�ɿ�Ĵָ��������һ������ı����������ռ���������������������������һ������˵��������������һ������Ŀ���Ƿ�ֹ��ȼʱ��������������ը��
Fe3O4+4H2���ڸ÷�Ӧ�У���ʧȥ���ӣ�������������ԭ����ˮ�е���Ԫ�ػ�õ��ӣ�����ԭ��������������2��ʵ��ǰ���������װ�ý��������Լ�飬���������ǽ�A�����ܲ���ˮ�У���������ƿ�����ܿ������ݣ���ȴ���γ�һ��ˮ������˵�������Ժã���3��Բ����ƿ��ʢװ��ˮ����װ�����Ⱥ����Ҫ�����Dz���ˮ������Ϊ����ˮ������Ӧ�ṩ��Ӧ���ƿ�ײ������˼�Ƭ���Ƭ�����Ƭ�������Ƿ�ֹ���У���4���ƾ��ƺ;ƾ���Ƶ�ȼ��˳�����ȵ�ȼ�ƾ��ƣ���װ���г���ˮ�������ٵ�ȼ�ƾ���ƣ�Ŀ���Ƿ�ֹ����������Ӧ����5���������ʢװ�ǵ������Ǽ�ʯ�ң����������������е�ˮ��������6���Թ����ռ����������������������ǿ�ȼ�Ե����壬����ڵ�ȼ֮ǰ��������鴿������������ȡ���Թܣ��ô�Ĵָ��ס�ܿڣ��ƽ��ƾ��ƵĻ��棬�ɿ�Ĵָ��������һ������ı����������ռ���������������������������һ������˵��������������һ������Ŀ���Ƿ�ֹ��ȼʱ��������������ը��

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�����Ŀ��ʵ���ҴӺ�������ȡ��IJ��������У�����ѡ�ò���ȷ���� ( )
ѡ�� | A | B | C | D |
���� | ��ȡ3g���ҵļ����˵ĸɺ��� | ���ոɺ�����Ƭ����ȫ��ɻҽ� | ������к�ĺ�������ˮ�Ļ��Һ | �����Ȼ�̼��������ĺ����ҽ�ȡҺ����ȡ�� |
ѡ������ | ������ƽ | ������ | ��ͨ©�� | ��Һ©�� |
A. A B. B C. C D. D