��Ŀ����
3��ʵ����Ҫ����100mL 0.5mol•L-1��NaCl��Һ���Իش����и��⣺��1�����������У��϶������õ�����ABC��
A����ƿ B��200mL����ƿ C����Ͳ D����ͷ�ι� E��100mL����ƿ F����ƽ
��2����Ҫʵʩ���ƣ������������⣬��ȱ�ٵIJ��������Dz���������ͷ�ιܣ�
��3������ƿ��ʹ��ǰ������е�һ�������Dz�©��
��4��������Ϻ�ʦָ������λͬѧ������������ijһ��������������Ϊ�������������ᵼ��������ҺŨ��ƫ�ߵ���B��
A������ʱ��������ƿ�̶���
B������ʱ��������ƿ�̶���
C�����ܽ���ȴ�����Һֱ��ת������ƿ��ͽ��ж��ݲ���
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ�
��5��ͨ������ɵó�����������ƽ��ȡNaCl����2.9�ˣ�����4mol/L��NaClŨ��Һ����100mL0.5mol•L-1��ϡ��Һ��Ӧ����Ͳ��ȡ12.5mL��Ũ��Һ��
���� ��1����2������ʵ���������ȷ��ÿ��������Ҫ������
��3�����������ߵ�ҡ�ȣ�ʹ������ƿʹ��ǰ�������Ƿ�©ˮ��
��4�������������������ʵ����ʵ�������Һ�������Ӱ�죬����C=$\frac{n}{V}$������������
��5������m=CVM������Ҫ�����Ȼ��Ƶ�������
����4mol/L��NaClŨ��Һ����100mL0.5mol•L-1��ϡ��Һ������ҪŨ��Һ���ΪV������ϡ��ǰ�����ʵ����ʵ����������V��
��� �⣺��1����2��������Һ�IJ������裺���ȼ������Ҫ��ҩƷ��������Ȼ����������ƽ������������ձ����ܽ⣬ͬʱ�ò��������裬����Һ��ȴ�����º��ò�����������Һ��100mL����ƿ��Ȼ��ϴ���ձ��Ͳ�����2��3�Σ���ϴ��ҺҲע������ƿ��Ȼ��������ƿ��עˮ����Һ����̶���1��2CMʱ�����ý�ͷ�ι���μ��룬����Һ����̶������У�Ȼ��ҡ�ȡ�װƿ���õ��������У�������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ��ò�����������A����ƿ B��200mL����ƿ C����Ͳ����ȱ�ٵIJ�������������������ͷ�ιܣ�
�ʴ�Ϊ��ABC������������ͷ�ιܣ�
��3�����������ߵ�ҡ�ȣ�ʹ������ƿʹ��ǰ�������Ƿ�©ˮ��
�ʴ�Ϊ����©��
��4��A������ʱ��������ƿ�̶��ߣ�������Һ�����ƫ����Һ��Ũ��ƫ�ͣ���A��ѡ��
B������ʱ��������ƿ�̶��ߣ�������Һ�����ƫС����Һ��Ũ��ƫ�ߣ���Bѡ��
C�����ܽ���ȴ�����Һֱ��ת������ƿ��ͽ��ж��ݲ�����δϴ���ձ����������������Ȼ���մ���ձ����벣�����ϣ���������ƿ���Ȼ��Ƶ�ʵ��������С����ҺŨ��ƫ�ͣ���C��ѡ��
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ���������Һ�����ƫ����Һ��Ũ��ƫ�ͣ���D��ѡ��
��ѡ��B��
��5������100mL 0.5mol•L-1��NaCl��Һ����Ҫ�����Ȼ��Ƶ�����=0.5mol•L-1��0.1L��58.5g/mol=2.9g��
����4mol/L��NaClŨ��Һ����100mL0.5mol•L-1��ϡ��Һ������ҪŨ��Һ���ΪV������ϡ��ǰ�����ʵ����ʵ��������0.5mol•L-1��0.1L=4mol/L��V����ã�V=0.0125L����12.5ml��
�ʴ�Ϊ��2.9��12.5��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ϥ����ԭ�������ǽ���ؼ���ע��������������C=$\frac{n}{V}$����Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| Ʒ�� | �������� |
| ����֭ | ��Ũ������֭ �ڰ�ɰ�� �����ʻ� ��ɽ����� |
| ���ȳ� | �ݾ�ѡ���� ��ʳ�� ��ζ�� ���������� |
| ������ | ��С��� ��ţ��⑪��ˮ����⑫����⑬����⑭ʳ������� |
| A�� | ������֮����1��1 | B�� | ԭ�Ӹ�������3��2 | C�� | ���������� 3��2 | D�� | �ܶ�֮����1��1 |
 ����Ӧ�����Һ�м���CCl4��Һ�������ú�ᷢ���²�Һ�����ɫΪ��ɫ���ٽ����Һ�����Һ©�������������ƣ��У�������Һ����룮
����Ӧ�����Һ�м���CCl4��Һ�������ú�ᷢ���²�Һ�����ɫΪ��ɫ���ٽ����Һ�����Һ©�������������ƣ��У�������Һ����룮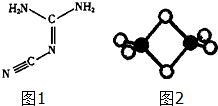 ˫�谷�ṹ��ʽ��ͼ1��
˫�谷�ṹ��ʽ��ͼ1��
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺ ��W�����ڱ��е�λ���ǵ�������VIA�壻
��W�����ڱ��е�λ���ǵ�������VIA�壻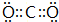 ��
��