��Ŀ����
����Ŀ����1�����ʵ���������Ϊb%��KOH��Һ������������ȥagˮ������������� 2b%�����ΪVmL�����ʱ��Һ�����ʵ���Ũ��Ϊ___________�����𰸱���Ϊ�����ʽ��
��2����6mol/L������(��=1.19g/cm3)50mLϡ�ͳ�3mol/L������(��=1.10g/cm3)�����ˮ�����Ϊ__________mL��
��3���ܶ�Ϊ1.19g/cm3�����ᣬ��������Ϊ25%���������õ������ˮϡ�ͺ�������Һ�����ʵ���������_______����������������С����������������12.5%��
��4����֪20��ʱ������NaCl��Һ���ܶ�Ϊ��g/cm3�����ʵ���Ũ��Ϊc mol/L����NaCl��Һ���ܽ��Ϊ_________g�����𰸱���Ϊ�����ʽ��
��5����ͬ������NO2��N2O4��������֮��Ϊ_______��������֮��Ϊ_______________��
��6�����õ����ű�ʾ��ѧ����ʽCaH2+2H2O=Ca(OH)2+2H2������ת�Ƶķ������Ŀ___________��
��7������������NaOH��Һ����NH4HCO3��Һ�У���Ӧ�Ļ�ѧ����ʽ__________________������NaOH��Һ���������NH4HCO3��Һ�У���Ӧ�����ӷ���ʽΪ__________________��
���𰸡� ![]() mol/L 50.5 ����
mol/L 50.5 ���� ![]() 1:1 1:1
1:1 1:1 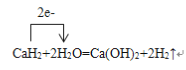 2NaOH+ 2NH4HCO3=Na2CO3+(NH4)2CO3+2H2O NH4++HCO3-+2OH-=CO32-+NH3��H2O+H2O
2NaOH+ 2NH4HCO3=Na2CO3+(NH4)2CO3+2H2O NH4++HCO3-+2OH-=CO32-+NH3��H2O+H2O
����������1����b%����������Һ������Ϊx������ǰ�����ʵ��������䣬��x��b%=��x-a����2b%����ã�x=2ag��2b%������������Һ�к����������ص����ʵ���Ϊ��![]() =
=![]() %mol��������Һ�����ʵ���Ũ��Ϊ��
%mol��������Һ�����ʵ���Ũ��Ϊ��![]() =
=![]() mol��L��1��
mol��L��1��
��2������ϡ�Ͷ��ɣ�ϡ��ǰ���������ʵ������䣬��ϡ�ͺ���������Ϊ50mL��6mol��L��1/3mol��L��1==100mL��Ũ���������Ϊ50mL��1.19g��mL��1=59.5g��ϡ���������Ϊ100mL��1.1g��mL��1=110g������ˮ������Ϊ110g-59.5g=50.5g������Ҫ����ˮ�����Ϊ50.5g/1g��mL��1=50.5mL��
��3����Ũ��������ΪV���ܶ�Ϊ�ѣ�Ũ����ϡ�ͺ��ܶ�Ϊ�ѣ�ϡ����ϡ��ǰ����Һ�����ʵ��������䣬��ϡ�ͺ�����������=![]() ��������ܶȴ���ˮ���ܶȣ�Ũ��Խ���ܶ�Խ�����Ԧѣ�Ũ��>�ѣ�ϡ��������
��������ܶȴ���ˮ���ܶȣ�Ũ��Խ���ܶ�Խ�����Ԧѣ�Ũ��>�ѣ�ϡ��������![]() >12.5%��
>12.5%��
��4��20��ʱ��1L����NaCl��Һ���ܽ���Ȼ��Ƶ�����Ϊ58.5cg����Һ����Ϊ1000�ѣ�����¶����Ȼ��Ƶ��ܽ��Ϊ��S=![]() ��100g=
��100g=![]() g��
g��
��5������ͬ������NO2��N2O4Ϊ1g��������֮��Ϊ![]() ��23��
��23��![]() ��46=1��1��������֮��=������֮��=1��1��
��46=1��1��������֮��=������֮��=1��1��
��6�����õ����ű�ʾ��ѧ����ʽCaH2+2H2O=Ca(OH)2+2H2������ת�Ƶķ������Ŀ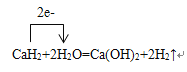
��7��HCO3��������ǿ��NH4����NaOH����HCO3����Ӧ������NH4����Ӧ������NaOH��Һ����������NH4HCO3��Һ�У�NaOHֻ��HCO3����Ӧ����Ӧ�Ļ�ѧ����ʽ2NaOH+ 2NH4HCO3=Na2CO3+(NH4)2CO3+2H2O������������NaOH��Һ����NH4HCO3��Һ�У���Ӧ�����ӷ���ʽΪNH4++HCO3-+2OH-=CO32-+NH3��H2O+H2O��

 ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
