题目内容
【题目】根据能量变化示意图,下列热化学方程式正确的是( )
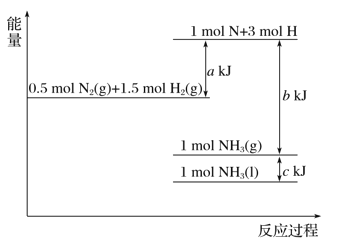
A. N2(g)+3H2(g)===2NH3(g) ΔH=-(b-a) kJ·mol-1
B. N2(g)+3H2(g)===2NH3(g) ΔH=-(a-b) kJ·mol-1
C. 2NH3(l)===N2(g)+3H2(g) ΔH=2(a+b-c) kJ·mol-1
D. 2NH3(l)===N2(g)+3H2(g) ΔH=2(b+c-a) kJ·mol-1
【答案】D
【解析】由图可知,![]() N2(g)+
N2(g)+![]() H2(g)=NH3(g)△H=(a-b)kJmol-1,
H2(g)=NH3(g)△H=(a-b)kJmol-1,![]() N2(g)+
N2(g)+![]() H2(g)=NH3(l)△H=(a-b-c)kJmol-1。A.N2(g)+3H2(g)=2NH3(g)△H=-2(b-a)kJmol-1,故A错误;B.N2(g)+3H2(g)=2NH3(g)△H═-2(b-a)kJmol-1,故B错误;C.物质的量与热量成正比、互为可逆反应的焓变的数值相同而符号相反,则2NH3(1)=N2(g)+3H2(g)△H=2(-a+b+c)kJmol-1,故C错误;D.结合选项C可知,2NH3(1)=N2(g)+3H2(g)△H=2(b+c-a)kJmol-1,故D正确;故选D。
H2(g)=NH3(l)△H=(a-b-c)kJmol-1。A.N2(g)+3H2(g)=2NH3(g)△H=-2(b-a)kJmol-1,故A错误;B.N2(g)+3H2(g)=2NH3(g)△H═-2(b-a)kJmol-1,故B错误;C.物质的量与热量成正比、互为可逆反应的焓变的数值相同而符号相反,则2NH3(1)=N2(g)+3H2(g)△H=2(-a+b+c)kJmol-1,故C错误;D.结合选项C可知,2NH3(1)=N2(g)+3H2(g)△H=2(b+c-a)kJmol-1,故D正确;故选D。

练习册系列答案
 口算小状元口算速算天天练系列答案
口算小状元口算速算天天练系列答案
相关题目