��Ŀ����
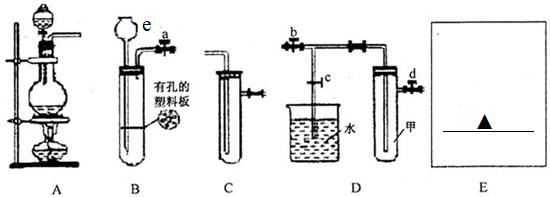
2�� ��������ƣ�Na2S2O3�������������Լ������ﻹԭ���������ȡ������ֽ⣮��ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2�T3Na2S2O3+CO2�Ƶã�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ����˵����a��ʢ��ϡ���b��ʢ��Na2SO3���壩�ش��������⣺
��������ƣ�Na2S2O3�������������Լ������ﻹԭ���������ȡ������ֽ⣮��ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2�T3Na2S2O3+CO2�Ƶã�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ����˵����a��ʢ��ϡ���b��ʢ��Na2SO3���壩�ش��������⣺��1������b��������������ƿ
��2��b�з�Ӧ�����ӷ���ʽΪ��SO32-+2H+=SO2��+H2O
��3����Ӧ��ʼ��c�����л��Dz��������ֱ���壮�˻�������S��
��4��d�е��Լ�Ϊ����������Һ�������ķ�Ӧ�ķ�Ӧ��2NaOH+SO2=Na2SO3+H2O��2NaOH+CO2=Na2CO3+H2O��2NaOH+H2S=Na2S+2H2O
��5��������Ҫ����SO2�������ʣ����Բ�ȡ�Ĵ�ʩ�п��Ʒ�Ӧ���¶Ȼ�����ĵμ��ٶȣ�д����������
��6��Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ���SO2���ܹ�����ԭ������ͨ���SO2����������Һ�����ԣ���������ƻ�ֽ⣮
���� ��һ��װ��Ϊ�����������ȡװ�ã���ȡ���������ԭ��Ϊ���������ƺ�70%��Ũ���cװ��ΪNa2S2O3������װ�ã�dװ��Ϊβ������װ�ã����ն��������������������壻��Ӧ��ʼʱ�����ķ�ӦΪ��Na2S+4SO2+H2O=2H2S+Na2SO3��SO2+2H2S=3S��+2H2O��
��1��װ���е�����b��������ƿ��
��2��b�з�Ӧ���Ʊ�������������ķ�Ӧ��װ��bΪ�����������ȡ����ȡ���������ԭ��Ϊ���������ƺ�70%��Ũ���cװ��ΪNa2S2O3������װ�ã�
��3����Ӧ��ʼ��c�����л��Dz��������ֱ���壬����SO2���������ԣ��������Ʒ���������ԭ��Ӧ���ɵ���S���Ӷ�ʹ��Һ����ǣ���˷�Ӧ��ʼ�����Ļ�������S��
��4��dװ��Ϊβ������װ�ã����ն��������������������壻
��5��ͨ�����Ʒ�Ӧ���¶Ȼ�����ĵμ��ٶȿ��Կ���SO2�������ʣ�
��6����������������ֽ⣬��ͨ���SO2����������Һ�����ԣ���������ƻ�ֽ⣮
��� �⣺��1��װ����b�����Ǵ�֧�ܵ���ƿΪ������ƿ������b��������������ƿ��
�ʴ�Ϊ��������ƿ��
��2����һ��װ��Ϊ�����������ȡװ�ã���ȡ���������ԭ��Ϊ���������ƺ�70%��Ũ���ᣬ��Ӧ�����ӷ���ʽΪ��SO32-+2H+=SO2��+H2O��
�ʴ�Ϊ��SO32-+2H+=SO2��+H2O��
��3����Ӧ��ʼʱ�����ķ�ӦΪ��Na2S+SO2+H2O=H2S+Na2SO3��SO2+2H2S=3S��+2H2O���ʸû�������S��
�ʴ�Ϊ��S��
��4��dװ��Ϊβ������װ�ã����ն�����������̼��������������壬Ӧѡ������������Һ����Ӧ�Ļ�ѧ����ʽΪ��2NaOH+SO2=Na2SO3+H2O 2NaOH+CO2=Na2CO3+H2O��2NaOH+H2S=Na2S+2H2O��
�ʴ�Ϊ������������Һ��2NaOH+SO2=Na2SO3+H2O 2NaOH+CO2=Na2CO3+H2O��2NaOH+H2S=Na2S+2H2O��
��5��ͨ�����Ʒ�Ӧ���¶Ȼ�����ĵμ��ٶȿ��Կ���SO2�������ʣ�
�ʴ�Ϊ�����Ʒ�Ӧ���¶Ȼ�����ĵμ��ٶȣ�
��6����������������ֽ⣬��ͨ���SO2����������Һ�����ԣ���������ƻ�ֽ⣬
�ʴ�Ϊ����ͨ���SO2����������Һ�����ԣ���������ƻ�ֽ⣮
���� ���⿼���������Ʊ�ʵ�鷽������ƺ��Ʊ����̷���Ӧ�ã����ջ�����ע������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| ���� | X | Y | Z |
| ��ʼŨ��/mol•L-1 | 0.1 | 0.2 | 0 |
| ƽ��Ũ��/mol•L-1 | 0.05 | 0.05 | 0.1 |
| A�� | ��Ӧ�ﵽƽ��ʱ��X��ת����Ϊ50% | |
| B�� | �ı��¶ȿ��Ըı�˷�Ӧ��ƽ�ⳣ�� | |
| C�� | ����ѹǿʹƽ��������Z�ķ����ƶ���ƽ�ⳣ������ | |
| D�� | ��Ӧ�ɱ�ʾΪX+3Y?2Z����ƽ�ⳣ��Ϊ1600 |
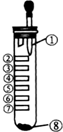 �������ʵ���ʵ��װ����ͼ��ʾ������Ũ���ᣬ���Ǹ�����أ�����������ֽ�������ε���Ʒ ����Һ��ʯ����Һ������KI��Һ��Na2S ��Һ��KBr ��Һ����KSCN ��FeCl2������Һ��ʵ��ʱ������Ũ���ᣬ����˵��������ǣ�������
�������ʵ���ʵ��װ����ͼ��ʾ������Ũ���ᣬ���Ǹ�����أ�����������ֽ�������ε���Ʒ ����Һ��ʯ����Һ������KI��Һ��Na2S ��Һ��KBr ��Һ����KSCN ��FeCl2������Һ��ʵ��ʱ������Ũ���ᣬ����˵��������ǣ�������| A�� | �������������ӷ���ʽ��16H++10Cl-+2MnO${\;}_{4}^{-}$=2Mn2++5Cl2��+8H2O | |
| B�� | �ߴ���Ѫ��ɫ������Ϊ2Fe2++Cl2=2Fe3++2Cl-��Fe3++3SCN-=Fe��SCN��3 | |
| C�� | ����ɫ���۴��ȱ�����ɫ���ݴ����ֵ���ɫ���� | |
| D�� | �ܴ�����������Ⱥ죬��˵�������ԣ�Cl2��Br2��I2 |
 2 ?+3 H2�� D��Al3++4 OH?��AlO2? +2H2O
2 ?+3 H2�� D��Al3++4 OH?��AlO2? +2H2O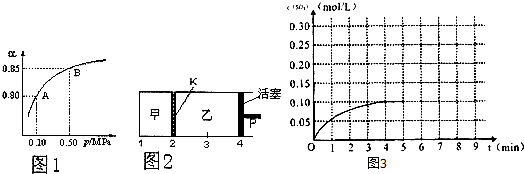
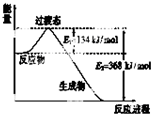 ��1����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��NO2��g��+CO��g��=NO��g��+CO2��g����H=-234 kJ•mol-1
��1����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��NO2��g��+CO��g��=NO��g��+CO2��g����H=-234 kJ•mol-1