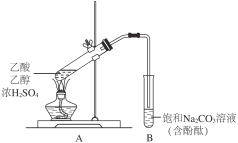
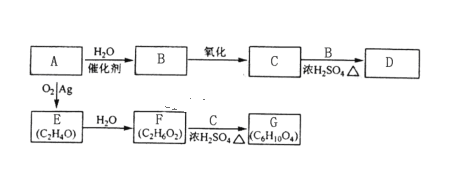
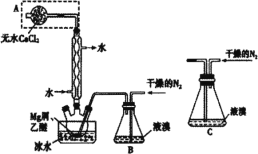
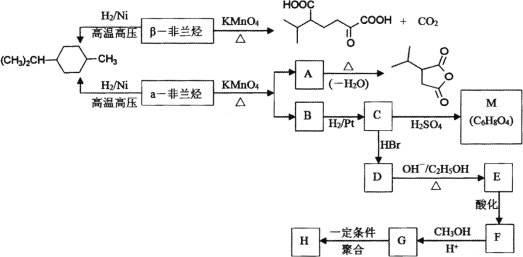
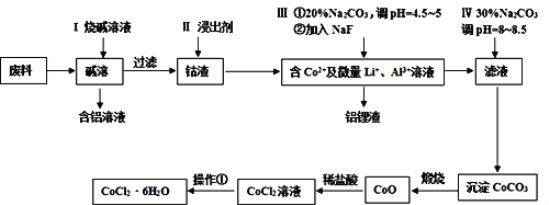
��Ŀ����
����Ŀ���ϳɰ��������ѧ������չʷ�ϵ�һ���ش�ͻ�ƣ��о�����Һ����һ�����õĴ������ʡ�
(1) �����ֽⷴӦ���Ȼ�ѧ����ʽ���£�2NH3(g) ![]() N2(g)��3H2(g)����H
N2(g)��3H2(g)����H
����N��N����H��H����N��H���ļ��ֱܷ����a��b��c(��λ��kJ��mol1)��������Ӧ����H��________kJ��mol1��
(2) �о��������������ɼ��ٰ����ķֽ⡣�±�Ϊij�¶��µ������IJ�ͬ�����ֱ����Ũ�Ȱ����ֽ����������ij�ʼ����(mmol��min1)��
���� | Ru | Rh | Ni | Pt | Pd | Fe |
��ʼ���� | 7.9 | 4.0 | 3.0 | 2.2 | 1.8 | 0.5 |
�ٲ�ͬ���������£������ֽⷴӦ���������________(��д�����Ļ�ѧʽ)��
���¶�ΪT����һ����̶����ܱ������м���2 mol NH3����ʱѹǿΪP0����Ru�������ֽ⣬��ƽ��ʱ�����ֽ��ת����Ϊ50%������¶��·�Ӧ2NH3(g) ![]() N2(g)��3H2(g)��ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp��________��[��֪�������ѹ(p��)��������ѹ(p��)���������]
N2(g)��3H2(g)��ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp��________��[��֪�������ѹ(p��)��������ѹ(p��)���������]
(3) ���ںϳɰ����յ����⣬������ȷ����________��
A���ϳɰ���ҵ�����õķ�Ӧ�¶�Ϊ500�����ң�������������ԭ������
B��ʹ�ó�ʼ��Ӧ���ʸ���Ĵ���Ru���������ƽ��ʱNH3�IJ���έ
C���ϳɰ���ҵ����10 MPa��30 MPa������ѹ��N2��H2��ת���ʲ���
D��������ˮ���µķ����ɽ��ϳɺ��������еİ�Һ��
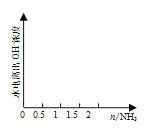
(4) ��1 L 1 mol��L1�������л���ͨ��2 mol����������ͼ�л�����Һ��ˮ�������OHŨ���氱��ͨ��仯������ͼ��______________________________
(5) �绯ѧ��Ҳ�ɺϳɰ�����ͼ���õ��¹������ӵ�����Ϊ����ʣ���PtC3N4�������������H2(g)��N2(g)�ϳ�NH3��ԭ��ʾ��ͼ��
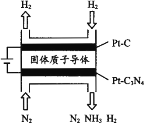
��PtC3N4�缫��Ӧ����NH3�ĵ缫��Ӧʽ________��
��ʵ���о�����������ӵ�ѹ����һ��ֵ�Ժ������������а���������������ŵ�ѹ���������С�����������ԭ��________��
���𰸡�6c��a��3b Fe ![]() BC
BC 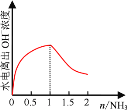 N2��6e��6H+=2NH3 ����һ����ѹ�Ժ�H+�õ��ӱ��H2���������ʱȵ�����
N2��6e��6H+=2NH3 ����һ����ѹ�Ժ�H+�õ��ӱ��H2���������ʱȵ�����
��������
��1��2NH3(g) ![]() N2(g)��3H2(g)�У�N��N��1����N��H��6����H-H��3������H=��Ӧ��ļ���֮����������ļ���֮��=6c��a��3b��
N2(g)��3H2(g)�У�N��N��1����N��H��6����H-H��3������H=��Ӧ��ļ���֮����������ļ���֮��=6c��a��3b��
��2�����������ɼ��ٰ����ķֽ⣬��ӦԽ����˵����Ӧ����Ļ��Խ�ݱ���֪��Fe��ʱ��Ӧ���������������ֽⷴӦ���������Fe��
��3����Ӧ2NH3(g) ![]() N2(g)��3H2(g)��ʼʱ��2 mol NH3����ʱѹǿΪP0��NH3��ת����Ϊ50%����Ӧƽ��ʱ��ϵ����1mol NH3��0.5mol N2��1.5mol H2�������ʵ���Ϊ3mol������ƽ��ʱ����ѹΪ
N2(g)��3H2(g)��ʼʱ��2 mol NH3����ʱѹǿΪP0��NH3��ת����Ϊ50%����Ӧƽ��ʱ��ϵ����1mol NH3��0.5mol N2��1.5mol H2�������ʵ���Ϊ3mol������ƽ��ʱ����ѹΪ![]() P0�������ķ�ѹΪ
P0�������ķ�ѹΪ![]() P0�������ķ�ѹΪ
P0�������ķ�ѹΪ![]() P0�������ķ�ѹΪ
P0�������ķ�ѹΪ![]() P0����Kp=
P0����Kp=![]() =
=![]() ��
��
��4��A. �ϳɰ��ķ�Ӧ�Ƿ��ȵģ����Ժϳɰ�ʱ�¶�Խ�ͣ�����ת����Խ�ߡ����¶ȹ�����Ӱ������Ļ��ԣ������¶�Ϊ500�棬����������ԭ��û�й�ϵ����A����
B. ʹ�ô������ܸı䷴Ӧ�̶ȣ��ʲ������ƽ��ʱNH3�IJ�����B��ȷ��
C. �ϳɰ���ҵ����10 MPa��30 MPa������ѹ��N2��H2��ת���ʲ��ߣ���C��ȷ��
D. ����������ˮ�����Բ��ܲ�����ˮ���µķ����ɽ��ϳɺ��������еİ�Һ������D����
��ȷ����BC��
��5���տ�ʼͨ��NH3��NH3��HCl��Ӧ�����Ȼ�泥��ٽ�ˮ�ĵ��룬��NH3��������1molʱ��HCl��Ӧ�꣬���ɰ�ˮ����������ˮ�ĵ��룬���Դ���ͼ��

��6��PtC3N4�缫���������õ��ӣ��缫����ʽΪ��N2��6e��6H+=2NH3������ӵ�ѹ����һ��ֵ�Ժ������������а���������������ŵ�ѹ���������С�����������ԭ���dz���һ����ѹ�Ժ�H+�õ��ӱ��H2���������ʱȵ����졣

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�����Ŀ������������[CH3CH(OH)COO]2Fe3H2O��Mr=288����һ��ʳ�õIJ�����������Ч���������ã�������ˮ�������������Ҵ��������ֽ⣬��ͨ��������̼��������Ӧ�Ƶá�
CH3CH(OH)COOH+FeCO3+2H2O=[CH3CH(OH)COO]2Fe3H2O+CO2��
FeCO3������ˮ���ױ�������4FeCO3+6H2O+O2=4Fe(OH)3+4CO2
��.�����������Ʊ���
ij��ȤС����FeCl2��NH4HCO3�Ʊ�FeCO3��װ��ʾ��ͼ��ͼ��
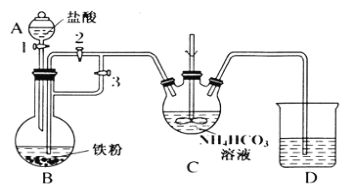
�ش��������⣺
(1)Cװ�����漰����Ҫ��Ӧ�����ӷ���ʽ_________��
(2)��D�������崿�������ɵ�FeCl2��Һ��NH4HCO3��Һ���ʱ�IJ�����_____��
(3)���Ƶõ�FeCO3���뵽����������Һ�У��ټ����������ۣ�75���½��跴Ӧ�������������۵�������_______��
(4)��Ӧ������������ˣ���ȥ�������۵ķ�����_________��
(5)��������Һ�л��������������ķ����ǣ�________����ȴ�ᾧ�����ˣ� �������Ҵ�ϴ�ӣ����
��.�����������崿�ȵIJ�����
(6)����ȤС����KMnO4�ζ����ⶨ��Ʒ�������������������Ʒ�������������������������ֲ�Ʒ�������������Ǵ���100%����ԭ�������___��
(7)������������ȤС�������(Ce)�����ⶨ��Ʒ��Fe2+�ĺ������ζ���Ӧ���£�Ce4++Fe2+=Ce3++Fe3+��ȡ1.440g��Ʒ���100mL��Һ��ÿ��ȡ20.00mL�����б�Ҫ��������0.0500molL-1Ce(SO4)2����Һ�ζ����յ㣬��¼���������
�ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
1 | 0.20 | 19.95 |
2 | 0.10 | 21.65 |
3 | 0.95 | 20.60 |
���Ʒ��������������������Ϊ________%��(С�������һλ����)
(8)�����ʵ��֤���㹺������������������к�Fe2+��_______��