ћвƒњƒЏ»Ё
°Њћвƒњ°њ±°Ї…”Ќ÷–Їђ”–…ўЅњµƒ¶ЅЈ«јЉћюЇЌ¶¬Ј«јЉћю£ђЅљ’яї•ќ™ЌђЈ÷“мєєће£ђ∆дѕаґ‘Ј÷„”÷ Ѕњќ™136°£ЄщЊЁ»зѕ¬„™їѓ£ђїЎірѕаєЎќ ћв£Ї
“—÷™£Ї
![]()
![]()
![]() £ЂRCHO
£ЂRCHO
![]()
![]()
![]() £ЂCO2
£ЂCO2
2CH3COOH![]()
![]()
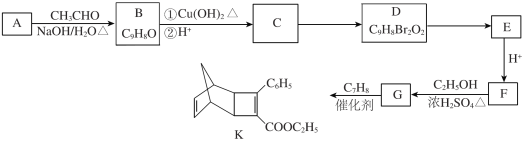
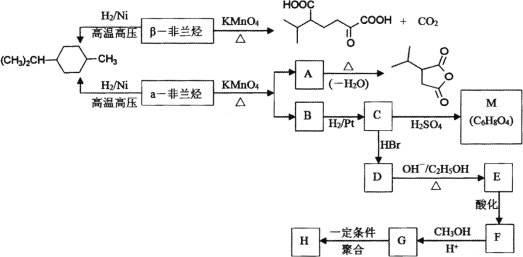
(1) –і≥ц¶ЅЈ«јЉћю÷–єўƒ№Ќ≈√ы≥∆________£ђ¶¬Ј«јЉћюµƒљбєєЉт љ________°£
(2) ѕ¬Ѕ–”–єЎЋµЈ®’э»Јµƒ «________°£
A£Ѓ¶ЅЈ«јЉћю”лµ»ќп÷ µƒЅњµƒBr2љш––Љ”≥…Јі”¶£ђ≤ъќпє≤”–3÷÷
B£ЃC°ъDЇЌE°ъFЈі”¶ја–ЌѕаЌђ
C£ЃЊџЇѕќпH“„»№”ЏЋЃ
D£ЃC°ъMЈі”¶єэ≥ћ÷–”–ЄяЈ÷„”ЊџЇѕќпµ»Є±≤ъќп≤ъ…ъ
(3) –і≥цF°ъGµƒїѓ—ІЈљ≥ћ љ________°£
(4) –і≥цЈыЇѕѕ¬Ѕ–ћхЉюµƒAµƒЌђЈ÷“мєєће________°£
ҐўЇђ”–4Єц£≠CH3£їҐЏ1 molіЋЌђЈ÷“мєєће‘ЏЉо–‘ћхЉюѕ¬ЋЃљв–и2 mol NaOH°£
(5) “‘Љ„±љЇЌ±ыѕ©ќ™їщ±Њ‘≠ЅѕЇѕ≥…![]() (”√Ѕч≥ћЌЉ±н Њ£ђ∆дЋыќёїъ ‘ЉЅ»ќ—°)________°£
(”√Ѕч≥ћЌЉ±н Њ£ђ∆дЋыќёїъ ‘ЉЅ»ќ—°)________°£
°Њір∞Є°њћЉћЉЋЂЉь ![]() AD CH2£љCHCOOH£ЂCH3OH
AD CH2£љCHCOOH£ЂCH3OH![]() CH2£љCHCOOCH3£ЂH2O
CH2£љCHCOOCH3£ЂH2O 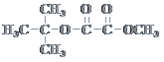 °°
°°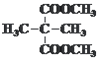 °°
°°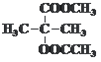 °°
°°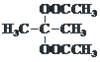
![]()
![]()
![]()
![]()
![]()
°Њљвќц°њ
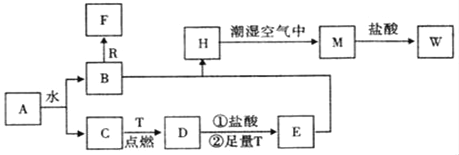
¶ЅЈ«јЉћюЈҐ…ъ–≈ѕҐ÷–—хїѓЈі”¶µ√µљA”лB£ђ”л«в∆шЈҐ…ъЉ”≥…Јі”¶µ√µљ![]() £ђAЉ”»»Ќ—ЋЃ…ъ≥…
£ђAЉ”»»Ќ—ЋЃ…ъ≥…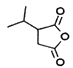 £ђ‘тAќ™
£ђ‘тAќ™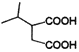 £ђљбЇѕAЇЌ
£ђљбЇѕAЇЌ![]() µƒљбєє£ђњ…Ќ∆÷™¶ЅЈ«јЉћюќ™
µƒљбєє£ђњ…Ќ∆÷™¶ЅЈ«јЉћюќ™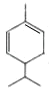 °ҐBќ™
°ҐBќ™![]() £ђB”л«в∆шЈҐ…ъЉ”≥…Јі”¶…ъ≥…Cќ™
£ђB”л«в∆шЈҐ…ъЉ”≥…Јі”¶…ъ≥…Cќ™![]() £ђC”лHBrЈҐ…ъ»°іъЈі”¶…ъ≥…Dќ™CH3CHBrCOOH£ђD‘Џ«в—хїѓƒ∆іЉ»№“Ї°ҐЉ”»»ћхЉюѕ¬ЈҐ…ъѕы»•Јі”¶°Ґ÷–ЇЌЈі”¶µ√µљEќ™CH2£љCHCOONa£ЃEЋбїѓµ√µљFќ™CH2£љCHCOOH£ђF”лЉ„іЉЈҐ…ъх•їѓЈі”¶µ√µљGќ™CH2£љCHCOOCH3£ђGЈҐ…ъЉ”ЊџЈі”¶µ√µљHќ™
£ђC”лHBrЈҐ…ъ»°іъЈі”¶…ъ≥…Dќ™CH3CHBrCOOH£ђD‘Џ«в—хїѓƒ∆іЉ»№“Ї°ҐЉ”»»ћхЉюѕ¬ЈҐ…ъѕы»•Јі”¶°Ґ÷–ЇЌЈі”¶µ√µљEќ™CH2£љCHCOONa£ЃEЋбїѓµ√µљFќ™CH2£љCHCOOH£ђF”лЉ„іЉЈҐ…ъх•їѓЈі”¶µ√µљGќ™CH2£љCHCOOCH3£ђGЈҐ…ъЉ”ЊџЈі”¶µ√µљHќ™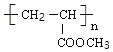 £Ѓ”…MµƒЈ÷„” љ£ђњ…÷™2Ј÷„”CЈҐ…ъх•їѓЈі”¶–ќ≥…їЈх•M£ђMµƒљбєєЉт љќ™
£Ѓ”…MµƒЈ÷„” љ£ђњ…÷™2Ј÷„”CЈҐ…ъх•їѓЈі”¶–ќ≥…їЈх•M£ђMµƒљбєєЉт љќ™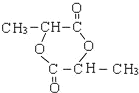 °£ЊЁіЋЈ÷ќцљвір°£
°£ЊЁіЋЈ÷ќцљвір°£
£®1£©¶ЅЈ«јЉћюЈҐ…ъ–≈ѕҐ÷–—хїѓЈі”¶µ√µљA”лB£ђ”л«в∆шЈҐ…ъЉ”≥…Јі”¶µ√µљ![]() £ђљбЇѕA°Ґ
£ђљбЇѕA°Ґ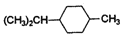 µƒљбєє£ђњ…Ќ∆÷™¶ЅЈ«јЉћюќ™
µƒљбєє£ђњ…Ќ∆÷™¶ЅЈ«јЉћюќ™ £ђєўƒ№Ќ≈ќ™£ЇћЉћЉЋЂЉь£ї
£ђєўƒ№Ќ≈ќ™£ЇћЉћЉЋЂЉь£ї![]()
![]()
![]() £ЂCO2£ђљбЇѕіЋјаЈі”¶њ…÷™¶¬Ј«јЉћюµƒљбєєЉт љќ™
£ЂCO2£ђљбЇѕіЋјаЈі”¶њ…÷™¶¬Ј«јЉћюµƒљбєєЉт љќ™![]() £ї
£ї
£®2£©A. Є√”–їъќп÷–ЅљЄцћЉћЉЋЂЉьі¶”ЏЉдќї£ђЋь”лµ»ќп÷ µƒЅњµƒBr2љш––Љ”≥…Јі”¶£ђ”–3÷÷Љ”≥…Јљ љ£ђЋщ“‘∆дЉ”≥…≤ъќп”–3÷÷£ђє A’э»Ј£ї
B. C°ъD «»°іъЈі”¶£ђE°ъF «ЋЃљвЈі”¶£ђє Bінќу£ї
C. ЊџЇѕќпH÷–√ї”–ф«їщїт’яф»їщ£ђ≤ї“„»№”ЏЋЃ£ђє Cінќу£ї
D. ”…MµƒЈ÷„” љ£ђњ…÷™2Ј÷„”CЈҐ…ъх•їѓЈі”¶–ќ≥…їЈх•M£ђMµƒљбєєЉт љќ™ £ђ
£ђ ![]() ÷–”–“їЄцф»їщ“їЄцф«їщ£ђњ…“‘ЈҐ…ъЊџЇѕЈі”¶£ђє D’э»Ј£їє —°AD°£
÷–”–“їЄцф»їщ“їЄцф«їщ£ђњ…“‘ЈҐ…ъЊџЇѕЈі”¶£ђє D’э»Ј£їє —°AD°£
£®3£©F”лЉ„іЉЈҐ…ъх•їѓЈі”¶µ√µљGќ™CH2£љCHCOOCH3£ђЈљ≥ћ љќ™CH2£љCHCOOH£ЂCH3OH![]() CH2£љCHCOOCH3£ЂH2O°£
CH2£љCHCOOCH3£ЂH2O°£
£®4£©1 molіЋЌђЈ÷“мєєће‘ЏЉо–‘ћхЉюѕ¬ЋЃљв–и2 mol NaOH£ђЋµ√ч”–їъќп÷––и“™”–ЅљЄцћЉ—хЋЂЉь£ђЌђЈ÷“мєєћеќ™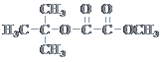 °°
°°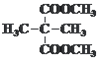 °°
°°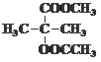 °°
°°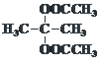 £ї
£ї
£®5£©“‘Љ„±љЇЌ±ыѕ©ќ™їщ±Њ‘≠ЅѕЇѕ≥…![]() £ђґ‘’’Љ„±љЇЌ≤ъќпµƒљбєє£ђљбЇѕЉ„±љµƒ–‘÷ £ђ…иЉ∆Ѕч≥ћЌЉќ™£Ї
£ђґ‘’’Љ„±љЇЌ≤ъќпµƒљбєє£ђљбЇѕЉ„±љµƒ–‘÷ £ђ…иЉ∆Ѕч≥ћЌЉќ™£Ї![]()
![]()
![]()
![]()
![]() °£
°£

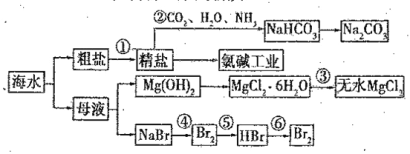
 љт«≈љћ”эЉ∆Ћг–°„і‘™ѕµЅ–ір∞Є
љт«≈љћ”эЉ∆Ћг–°„і‘™ѕµЅ–ір∞Є°Њћвƒњ°њ“—÷™£ЇAlO£ЂHCO£ЂH2O=Al(OH)3°э£ЂCO![]() £ђѕтЇђ0.01 mol NaAlO2ЇЌ0.02mol NaOHµƒѕ°»№“Ї÷–їЇ¬эЌ®»лґю—хїѓћЉ£ђЋжn(CO2)‘ціу£ђѕ»ЇуЈҐ…ъ»эЄц≤їЌђµƒЈі”¶£ђѕ¬Ѕ–ґ‘”¶єЎѕµ’э»Јµƒ «£® £©
£ђѕтЇђ0.01 mol NaAlO2ЇЌ0.02mol NaOHµƒѕ°»№“Ї÷–їЇ¬эЌ®»лґю—хїѓћЉ£ђЋжn(CO2)‘ціу£ђѕ»ЇуЈҐ…ъ»эЄц≤їЌђµƒЈі”¶£ђѕ¬Ѕ–ґ‘”¶єЎѕµ’э»Јµƒ «£® £©
—°ѕо | n(CO2)/mol | »№“Ї÷–јл„”µƒќп÷ µƒЅњ≈®ґ» |
A | 0 | c(Na+)£Њc(AlO2°™)£Њc(OH) |
B | 0.01 | c(CO32°™)£Ђc(HCO3°™)£Ђc(H2CO3)£љc(AlO2°™) |
C | 0.015 | c(Na+)£Њc(CO32°™)£Њc(OH)£Њc(HCO3°™) |
D | 0.03 | c(Na+)£Ђc(H+)£љc(CO32°™)£Ђc(HCO3°™)£Ђc(OH) |
A.AB.BC.CD.D
°Њћвƒњ°њЇѕ≥…∞± «»Ћјањ∆—ІЉЉ хЈҐ’є Ј…ѕµƒ“їѕо÷ЎіуЌї∆∆£ђ—–Њњ±н√ч“Ї∞± «“ї÷÷ЅЉЇ√µƒіҐ«вќп÷ °£
(1) ∞±∆шЈ÷љвЈі”¶µƒ»»їѓ—ІЈљ≥ћ љ»зѕ¬£Ї2NH3(g) ![]() N2(g)£Ђ3H2(g)°°¶§H
N2(g)£Ђ3H2(g)°°¶§H
»ф£ЇN°‘NЉь°ҐH£≠HЉьЇЌN£≠HЉьµƒЉьƒ№Ј÷±рЉ«„чa°ҐbЇЌc(µ•ќї£ЇkJ°§mol1)‘т…ѕ цЈі”¶µƒ¶§H£љ________kJ°§mol1°£
(2) —–Њњ±н√чљр фіяїѓЉЅњ…Љ”Ћў∞±∆шµƒЈ÷љв°£ѕ¬±нќ™ƒ≥ќ¬ґ»ѕ¬µ»÷ Ѕњµƒ≤їЌђљр фЈ÷±ріяїѓµ»≈®ґ»∞±∆шЈ÷љв…ъ≥…«в∆шµƒ≥х ЉЋў¬ (mmol°§min1)°£
іяїѓЉЅ | Ru | Rh | Ni | Pt | Pd | Fe |
≥х ЉЋў¬ | 7.9 | 4.0 | 3.0 | 2.2 | 1.8 | 0.5 |
Ґў≤їЌђіяїѓЉЅіж‘Џѕ¬£ђ∞±∆шЈ÷љвЈі”¶їоїѓƒ№„оіуµƒ «________(ћо–ііяїѓЉЅµƒїѓ—І љ)°£
ҐЏќ¬ґ»ќ™T£ђ‘Џ“їћеїэєћґ®µƒ√№±’»Ё∆ч÷–Љ”»л2 mol NH3£ђіЋ ±—є«њќ™P0£ђ”√Ruіяїѓ∞±∆шЈ÷љв£ђ»ф∆љЇв ±∞±∆шЈ÷љвµƒ„™їѓ¬ ќ™50%£ђ‘тЄ√ќ¬ґ»ѕ¬Јі”¶2NH3(g) ![]() N2(g)£Ђ3H2(g)”√∆љЇвЈ÷—єіъћж∆љЇв≈®ґ»±н Њµƒїѓ—І∆љЇв≥£ эKp£љ________°£[“—÷™£Ї∆шћеЈ÷—є(pЈ÷)£љ∆шће„№—є(p„№)°ЅћеїэЈ÷ э]
N2(g)£Ђ3H2(g)”√∆љЇвЈ÷—єіъћж∆љЇв≈®ґ»±н Њµƒїѓ—І∆љЇв≥£ эKp£љ________°£[“—÷™£Ї∆шћеЈ÷—є(pЈ÷)£љ∆шће„№—є(p„№)°ЅћеїэЈ÷ э]
(3) єЎ”ЏЇѕ≥…∞±є§“’µƒјнљв£ђѕ¬Ѕ–’э»Јµƒ «________°£
A£ЃЇѕ≥…∞±є§“µ≥£≤…”√µƒЈі”¶ќ¬ґ»ќ™500°ж„у”“£ђњ…”√ј’ѕƒћЎЅ–‘≠јнљв Ќ
B£Ѓ є”√≥х ЉЈі”¶Ћў¬ ЄьњмµƒіяїѓЉЅRu£ђ≤їƒ№ћбЄя∆љЇв ±NH3µƒ≤ъЅњќ≠
C£ЃЇѕ≥…∞±є§“µ≤…”√10 MPa°Ђ30 MPa£ђ «“т≥£—єѕ¬N2ЇЌH2µƒ„™їѓ¬ ≤їЄя
D£Ѓ≤…”√јдЋЃљµќ¬µƒЈљЈ®њ…љЂЇѕ≥…ЇуїмЇѕ∆шће÷–µƒ∞±“Їїѓ
(4) ‘Џ1 L 1 mol°§L1µƒ—ќЋб÷–їЇїЇЌ®»л2 mol∞±∆ш£ђ«л‘ЏЌЉ÷–ї≠≥ц»№“Ї÷–ЋЃµзјл≥цµƒOH≈®ґ»Ћж∞±∆шЌ®»л±дїѓµƒ«ч ∆ЌЉ°£______________________________
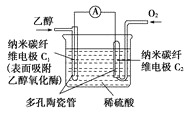
(5) µзїѓ—ІЈ®“≤њ…Їѕ≥…∞±°£ѕ¬ЌЉ «”√µЌќ¬єћће÷ „”µЉће„чќ™µзљв÷ £ђ”√PtC3N4„ч“хЉЂіяїѓЉЅµзљвH2(g)ЇЌN2(g)Їѕ≥…NH3µƒ‘≠јн Њ“вЌЉ£Ї
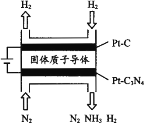
ҐўPtC3N4µзЉЂЈі”¶≤ъ…ъNH3µƒµзЉЂЈі”¶ љ________°£
ҐЏ µ—й—–Њњ±н√ч£ђµ±ЌвЉ”µз—є≥ђєэ“їґ®÷µ“‘Їу£ђЈҐѕ÷“хЉЂ≤ъќп÷–∞±∆шµƒћеїэЈ÷ эЋж„≈µз—єµƒ‘ціуґшЉх–°£ђЈ÷ќц∆дњ…ƒ№‘≠“т________°£