��Ŀ����
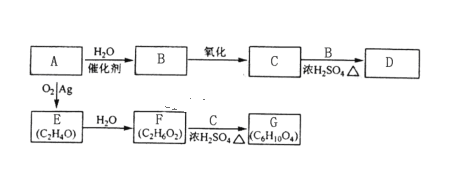
����Ŀ��A��ʯ���ѽ�������Ҫ�ɷ֣����IJ���ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ����Aԭ�����������ֻ�����Ʒ�ķ�Ӧ�������£����ַ�Ӧ��������ȥ����
��֪����2RCHO+O2![]() 2RCOOH����R-CH��CH-OHϩ��ʽ�Ľṹ�����ȶ����ڡ���ش��������⣺
2RCOOH����R-CH��CH-OHϩ��ʽ�Ľṹ�����ȶ����ڡ���ش��������⣺
��1��A�ĵ���ʽΪ___��
��2��B��D�����еĹ��������Ʒֱ���____��____��
��3��C��B��Ӧ����D�Ļ�ѧ����ʽΪ________��
��4��E�Ľṹ��ʽΪ_____��
��5��������⣬д��E���ܵ�ͬ���칹��Ľṹ��ʽ_____��
��6��F��C��Ӧ����G�Ļ�ѧ����ʽΪ_____��
���𰸡�![]() �ǻ� ���� CH3COOH+CH3CH2OH
�ǻ� ���� CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O ![]() CH3CHO 2CH3COOH+HOCH2CH2OH
CH3CHO 2CH3COOH+HOCH2CH2OH![]() CH3COOCH2CH2OOCCH3+2H2O
CH3COOCH2CH2OOCCH3+2H2O
��������
A��ʯ���ѽ�������Ҫ�ɷ֣����IJ���ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪ��ϩ����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ���BΪ�Ҵ����Ҵ��������ɵ����ʿ����Ҵ���Ӧ����CΪ���ᣬDΪ������������ϩ��������Ӧ���ɵIJ������ˮ������Ӧ����FΪ�Ҷ�����EΪ�������飻
��1��������֪AΪ��ϩ������ʽΪ![]() ��
��
��2��B��D�ֱ�Ϊ�Ҵ����������������еĹ����ŷֱ�Ϊ�ǻ���������
��3��B��C��D�ֱ�Ϊ�Ҵ������ᡢ�����������Ҵ������ᷴӦ��������������ˮ������ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��4��EΪ�������飬�ṹ��ʽΪ![]() ��
��
��5��E�ķ���ʽΪC2H4O��ͬ���칹��Ľṹ��ʽΪCH3CHO��
��6���Ҷ�����2����������Ũ������ȵ�����������CH3COOCH2CH2OOCCH3��ˮ������ʽΪ2CH3COOH+HOCH2CH2OH![]() CH3COOCH2CH2OOCCH3+2H2O��
CH3COOCH2CH2OOCCH3+2H2O��

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�����Ŀ���⼰�仯����������������Ӧ�ù㷺���ش���������:
��1�����ʵ������Ӧ�ɵ�IF5��ʵ�����Һ̬IF5����һ���ĵ�����ԭ������IF5����ż���루��:2H2O![]() H3O++OH-����IF5����ż���뷽��ʽΪ_________
H3O++OH-����IF5����ż���뷽��ʽΪ_________
��2���������ƺ͵������������Һ�еķ�Ӧ��:Na2SO3+KIO3+H2SO4 ��Na2SO4+K2SO4+I2+H2O��δ��ƽ���÷�Ӧ���̺ͻ����ϸ��ӣ�һ����Ϊ��Ϊ���²�:
��IO3-+SO32-��IO2-+SO42-������
��IO2-+SO32-��IO-+SO42-���죩
��5I-+6H++I03-��3I2+3H2O���죩
��I2+SO32-+H2O-��2I+SO42-+2H+���죩
�������������Ʋ⣬�˷�Ӧ���ܷ�Ӧ������_______����Ӧ����������ţ�����Ԥ�ȼ��˵�����Һ��������������_____________����������ʱ���Ż��е��۱��������������
��3�����ӵ���������Ҫ���о�����
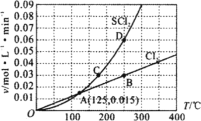
����֪SO2��I2�ķ�Ӧ�����ʼ�����ƽ�ⳣ������Һ�д�������ƽ��:I2(aq)+l-(aq)=l3-(aq)�ֽ�1 mol SO2��ͨ�뺬1mol l2��ˮ��Һ����ǡ����ȫ��Ӧ��Һ��l3-�����ʵ���n(l3-)ʱ�䣨t���ı仯������ͼ1��ʾ����ʼ�Σ�n(l3-)�������ԭ����_____
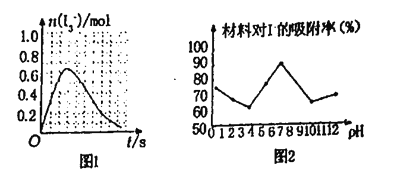
�ڿ���С�������Ͳ���Ag/TiO2����Һ�е����ӽ��������о�����ͼ2�Dz�ͬpH�����£�����������Ч���ı仯���ߡ��ݴ��ƶ�Ag/TiO2�������ʺ�����___________����������������������������������Һ�е�I-��
���Ȼ�������������Ҳ����Ч�����������Ȼ��������������Ե����ӵ�������ӦΪ:I-(aq)+AgCl(s)=Agl(s)+Cl-(aq)����Ӧ�ﵽƽ�����Һ��c(Cl-)=1.0mol��L-1.����Һ��c(I-)=______mol��L-1��k(AgCl)=2.0��10-10��k(Agl)=8.4��10-17��
��4��Fe3+��I-����Һ�з�����Ӧ:2Fe3++2I- ![]() 2Fe2++I2���÷�Ӧ������Ӧ���ʺ�Fe2+��I-��Ũ�ȹ�ϵΪv=k��cm(I-)��cn(Fe3+)������kΪ��������T��ʱ��ʵ����c��I-����c��Fe3+���뷴Ӧ���ʵĹ�ϵ���±���
2Fe2++I2���÷�Ӧ������Ӧ���ʺ�Fe2+��I-��Ũ�ȹ�ϵΪv=k��cm(I-)��cn(Fe3+)������kΪ��������T��ʱ��ʵ����c��I-����c��Fe3+���뷴Ӧ���ʵĹ�ϵ���±���
c��I-��/molL-1 | c��Fe3+��/molL-1 | v/molL-1s-1 | |
�� | 0.20 | 0.80 | 0.032k |
�� | 0.60 | 0.40 | 0.144k |
�� | 0.80 | 0.20 | 0.128k |
����v=k��cm(I-)��cn(Fe3+)��m��n��ֵΪ_____________������ţ�
A.m=1n=1 B.m=1��n=2 C.m=2��n=1 D.m=2��n=2
��I-Ũ�ȶԷ�Ӧ���ʵ�Ӱ��_____����������������С����������������Fe3+Ũ�ȶԷ�Ӧ���ʵ�Ӱ�졣
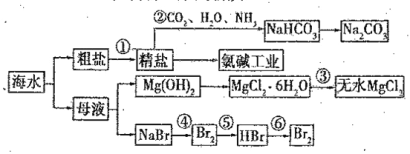
����Ŀ����֪��AlO��HCO��H2O=Al(OH)3����CO![]() ����0.01 mol NaAlO2��0.02mol NaOH��ϡ��Һ�л���ͨ�������̼����n(CO2)�����Ⱥ���������ͬ�ķ�Ӧ�����ж�Ӧ��ϵ��ȷ���ǣ� ��
����0.01 mol NaAlO2��0.02mol NaOH��ϡ��Һ�л���ͨ�������̼����n(CO2)�����Ⱥ���������ͬ�ķ�Ӧ�����ж�Ӧ��ϵ��ȷ���ǣ� ��
ѡ�� | n(CO2)/mol | ��Һ�����ӵ����ʵ���Ũ�� |
A | 0 | c(Na+)��c(AlO2��)��c(OH) |
B | 0.01 | c(CO32��)��c(HCO3��)��c(H2CO3)��c(AlO2��) |
C | 0.015 | c(Na+)��c(CO32��)��c(OH)��c(HCO3��) |
D | 0.03 | c(Na+)��c(H+)��c(CO32��)��c(HCO3��)��c(OH) |
A.AB.BC.CD.D
����Ŀ���ϳɰ��������ѧ������չʷ�ϵ�һ���ش�ͻ�ƣ��о�����Һ����һ�����õĴ������ʡ�
(1) �����ֽⷴӦ���Ȼ�ѧ����ʽ���£�2NH3(g) ![]() N2(g)��3H2(g)����H
N2(g)��3H2(g)����H
����N��N����H��H����N��H���ļ��ֱܷ����a��b��c(��λ��kJ��mol1)��������Ӧ����H��________kJ��mol1��
(2) �о��������������ɼ��ٰ����ķֽ⡣�±�Ϊij�¶��µ������IJ�ͬ�����ֱ����Ũ�Ȱ����ֽ����������ij�ʼ����(mmol��min1)��
���� | Ru | Rh | Ni | Pt | Pd | Fe |
��ʼ���� | 7.9 | 4.0 | 3.0 | 2.2 | 1.8 | 0.5 |
�ٲ�ͬ���������£������ֽⷴӦ���������________(��д�����Ļ�ѧʽ)��
���¶�ΪT����һ����̶����ܱ������м���2 mol NH3����ʱѹǿΪP0����Ru�������ֽ⣬��ƽ��ʱ�����ֽ��ת����Ϊ50%������¶��·�Ӧ2NH3(g) ![]() N2(g)��3H2(g)��ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp��________��[��֪�������ѹ(p��)��������ѹ(p��)���������]
N2(g)��3H2(g)��ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp��________��[��֪�������ѹ(p��)��������ѹ(p��)���������]
(3) ���ںϳɰ����յ����⣬������ȷ����________��
A���ϳɰ���ҵ�����õķ�Ӧ�¶�Ϊ500�����ң�������������ԭ������
B��ʹ�ó�ʼ��Ӧ���ʸ���Ĵ���Ru���������ƽ��ʱNH3�IJ���έ
C���ϳɰ���ҵ����10 MPa��30 MPa������ѹ��N2��H2��ת���ʲ���
D��������ˮ���µķ����ɽ��ϳɺ��������еİ�Һ��
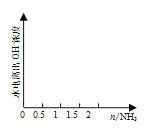
(4) ��1 L 1 mol��L1�������л���ͨ��2 mol����������ͼ�л�����Һ��ˮ�������OHŨ���氱��ͨ��仯������ͼ��______________________________
(5) �绯ѧ��Ҳ�ɺϳɰ�����ͼ���õ��¹������ӵ�����Ϊ����ʣ���PtC3N4�������������H2(g)��N2(g)�ϳ�NH3��ԭ��ʾ��ͼ��
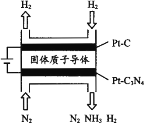
��PtC3N4�缫��Ӧ����NH3�ĵ缫��Ӧʽ________��
��ʵ���о�����������ӵ�ѹ����һ��ֵ�Ժ������������а���������������ŵ�ѹ���������С�����������ԭ��________��