��Ŀ����
1����1��ij�������A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫�����˴Ź������ױ���������ֻ��һ�����͵��⣮��A�Ľṹ��ʽΪ
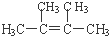 ��
����A�е�̼ԭ���Ƿ���ͬһƽ�棿�� ����ǡ����ߡ����ǡ�����
��2��ij�����廯����A��C8H8O2��
����A�����Լ����������¾���ˮ�⣬���ϴ�������A��ͬ���칹������������֣�


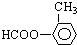 ����
����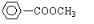 ��
��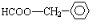 ��
��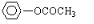
����A����NaHCO3��Ӧ�������壬��A���ܵĽṹ��4�֣�
���� ��1����A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫������AӦΪϩ���������ʽΪCnH2n������14n=84��n=6������Ϊ�˴Ź������ױ���������ֻ��һ�����͵��⣬��ṹ��ʽӦΪ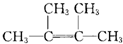 ��
��
�ڸ�����ϩ�ṹ���ص��жϣ�
��2���������������ºͼ��������¾���ˮ�⣬�ʺ���������
������̼�����Ʒ�Ӧ�������壬�����Ȼ����ݴ˽�ɣ�
��� �⣺��1����A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫������AӦΪϩ���������ʽΪCnH2n������14n=84��n=6������Ϊ�˴Ź������ױ���������ֻ��һ�����͵��⣬��ṹ��ʽӦΪ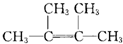 ���ʴ�Ϊ��
���ʴ�Ϊ��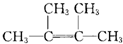 ��
��
������̼̼˫����ƽ���ͽṹ����4�����벻����̼ԭ����ֱ������������6��̼ԭ�Ӵ���ͬһƽ���ϣ��ʴ�Ϊ���ǣ�
��2����A�����Լ����������¾���ˮ�⣬����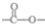 ��A�����DZ��������������ţ�ΪHCOO-��-CH3��HOOCCH2-��-COOCH3��CH3COO-���ʴ�Ϊ��
��A�����DZ��������������ţ�ΪHCOO-��-CH3��HOOCCH2-��-COOCH3��CH3COO-���ʴ�Ϊ�� ��
��
�ڷ����廯����A�����DZ��������������ţ������Ȼ�����Ϊ-COOH��-CH3���ڼ�����֣�ΪCH3COOH�����ܹ�4�֣��ʴ�Ϊ��4��
���� ���⿼�����л�����ƶϣ�ȷ��A�Ľṹ�ǹؼ����ٳ�����÷�Ӧ���������ƶϣ���ȷ�л���Ĺ�����������ת���ǹؼ����Ѷ��еȣ�

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�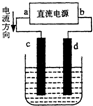
| A�� | aΪ������bΪ���� | B�� | c�缫�Ϸ�����ԭ��Ӧ | ||
| C�� | �������У�d�缫�������� | D�� | �������У�������Ũ�Ȳ��� |
| A�� | ���ķǽ������� | B�� | �������۷е�� | ||
| C�� | ��ԭ�Ӱ뾶��С | D�� | ��������ԭ�Ӽ乲�ۼ������� |
| A�� | 200 mL 2 mol•L-1HCl | B�� | 100 mL 2 mol•L-1H2SO4 | ||
| C�� | 100 mL 3 mol•L-1 HCl | D�� | 50 mL 18.4 mol•L-1H2SO4 |
| A�� | �ǽ����Ա���ǿ | B�� | ���������Ļ�ѧʽ��SeO2 | ||
| C�� | ���ԣ�H2SeO4��H2SO4 | D�� | ��̬�⻯��Ļ�ѧʽΪH2Se |
| A�� | 0.8a g | B�� | 0.0745b g | C�� | 0.0376c g | D�� | ������ |
| A�� | ȡ������Һ�������������ữ���Ȼ�����Һ���а�ɫ�������� | |
| B�� | ȡ������Һ�������Ȼ�����Һ���а�ɫ�������ɣ��ټ�ϡ�����������ʧ | |
| C�� | ȡ������Һ���������ᱵ��Һ���а�ɫ�������� | |
| D�� | ȡ������Һ�����������������ټ��Ȼ�����Һ���а�ɫ�������� |
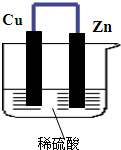 ��ͼ��ʾ����п��ͭͨ����������������ϡ�����У�
��ͼ��ʾ����п��ͭͨ����������������ϡ�����У�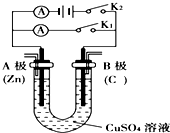 �밴Ҫ��ش��������⣮
�밴Ҫ��ش��������⣮