ΧβΡΩΡΎ»ί
3Θ°Θ®1Θ©“Σ≥ΐ»Ξ»γ±μΥυΝ–Έο÷ ÷–ΒΡ‘”÷ Θ®ά®Κ≈ΡΎΈΣ‘”÷ Θ©Θ§¥”Θ®IΘ©÷–―Γ‘ώ “ΥΒΡ ‘ΦΝΘ§¥”Θ®IIΘ©÷–―Γ‘ώΖ÷άκΧα¥ΩΒΡΖΫΖ®Θ§”Ο–ρΚ≈Χν»κ±μ÷–Θ°Θ®ΔώΘ© ‘ΦΝΘΚaΘ°≈®ΝρΥα bΘ°«β―θΜ·ΡΤ cΘ°…ζ ·Μ“ dΘ°Υ° eΘ°≈®δεΥ°
Θ®ΔρΘ©ΖΫΖ®ΘΚAΘ°œ¥Τχ BΘ°Ζ÷“Κ CΘ°’τΝσ
| “ΣΧα¥ΩΒΡΈο÷ Θ®‘”÷ Θ© | ““¥Φ Θ®Υ°Θ© | ““»≤Θ®H2SΘ© | δε±Ϋ Θ®δεΘ© |
| ―Γ”Ο ‘ΦΝ | |||
| Ζ÷άκΖΫΖ® | 1 |
Θ®3Θ©Ψέ¬»““œ© «…ζΜν÷–≥Θ”ΟΒΡΥήΝœΘ°ΙΛ“Β…ζ≤ζΨέ¬»““œ©ΒΡ“Μ÷÷ΙΛ“’¬ΖœΏ»γœ¬ΘΚ
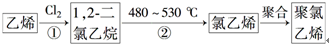
Ζ¥”ΠΔΌΒΡΜ·―ßΖΫ≥Χ ΫΈΣH2C=CH2+Cl2ΓζCH2ClCH2ClΘ§Ζ¥”Πάύ–ΆΈΣΦ”≥…Ζ¥”ΠΘ§Ζ¥”ΠΔΎΒΡΖ¥”Πάύ–ΆΈΣœϊ»ΞΖ¥”ΠΘ°
Θ®4Θ©Ρ≥”–ΜζΈο÷–Ης‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐ «ΘΚΧΦ49.5%Θ§«β5.20%Θ§―θ16.5%Θ§ΒΣ28.9%Θ§ΗΟ”–ΜζΈοΒΡ Β―ι ΫΈΣC4H5ON2Θ°
Ζ÷Έω Θ®1Θ©Υ°”κCaOΖ¥”ΠΚσΘ§‘ω¥σ”κ““¥ΦΒΡΖ–Βψ≤ν“λΘΜ
ΝρΜ·«β”κNaOH»ή“ΚΖ¥”ΠΘ§Εχ““»≤≤ΜΡήΘΜ
δε”κNaOH»ή“ΚΖ¥”ΠΚσΘ§”κδε±ΫΖ÷≤ψΘΜ
Θ®2Θ©BΈΣΖ÷“ΚΘ§±Ί“ΣΒΡ“«ΤςΈΣΖ÷“Κ¬©ΕΖΘΜ
Θ®3Θ©““œ©”ꬻΤχΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…1Θ§2-Ε଻““ΆιΘ§Φ”»»480ΓΪ530Γφ…ζ≥…¬»““œ©Θ§»ΜΚσΖΔ…ζΦ”ΨέΖ¥”ΠΩ……ζ≥…Ψέ¬»““œ©ΘΜ
Θ®4Θ©”…$\frac{C%}{12}$ΘΚ$\frac{H%}{1}$ΘΚ$\frac{O%}{16}$ΘΚ$\frac{N%}{14}$»ΖΕ®‘≠Ή”Ηω ΐ±»Θ§Φ¥Ω…ΒΟΒΫ Β―ι ΫΘ°
Ϋβ¥π ΫβΘΚΘ®1Θ©Υ°”κCaOΖ¥”ΠΚσΘ§‘ω¥σ”κ““¥ΦΒΡΖ–Βψ≤ν“λΘ§‘ρ ‘ΦΝΈΣcΘ§Ζ÷άκΖΫΖ®ΈΣCΘΜ
ΝρΜ·«β”κNaOH»ή“ΚΖ¥”ΠΘ§Εχ““»≤≤ΜΡήΘ§‘ρ ‘ΦΝΈΣbΘ§Ζ÷άκΖΫΖ®ΈΣAΘΜ
δε”κNaOH»ή“ΚΖ¥”ΠΚσΘ§”κδε±ΫΖ÷≤ψΘ§»ΜΚσΖ÷“ΚΩ…Ζ÷άκΘ§‘ρ ‘ΦΝΈΣbΘ§Ζ÷άκΖΫΖ®ΈΣBΘ§
Ι ¥πΑΗΈΣΘΚ
| “ΣΧα¥ΩΒΡΈο÷ Θ®‘”÷ Θ© | ““¥Φ Θ®Υ°Θ© | ““»≤Θ®H2SΘ© | δε±Ϋ Θ®δεΘ© |
| ―Γ”Ο ‘ΦΝ | c | b | b |
| Ζ÷άκΖΫΖ® | C | A | B |
Θ®2Θ©BΈΣΖ÷“ΚΘ§±Ί“ΣΒΡ“«ΤςΈΣΖ÷“Κ¬©ΕΖΘ§ΜΙ–η“Σ…’±≠Θ§Ι ¥πΑΗΈΣΘΚΖ÷“Κ¬©ΕΖΓΔ…’±≠ΘΜ
Θ®3Θ©““œ©”ꬻΤχΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…1Θ§2-Ε଻““ΆιΘ§Ζ¥”ΠΒΡΖΫ≥Χ ΫΈΣH2C=CH2+Cl2ΓζCH2ClCH2ClΘ§Φ”»»480ΓΪ530Γφ…ζ≥…¬»““œ©Θ§ΖΔ…ζœϊ»ΞΖ¥”ΠΘ§
Ι ¥πΑΗΈΣΘΚH2C=CH2+Cl2ΓζCH2ClCH2ClΘΜΦ”≥…Ζ¥”ΠΘΜœϊ»ΞΖ¥”ΠΘΜ
Θ®4Θ©ΧΦ49.5%Θ§«β5.20%Θ§―θ16.5%Θ§ΒΣ28.9%Θ§‘ρ$\frac{C%}{12}$ΘΚ$\frac{H%}{1}$ΘΚ$\frac{O%}{16}$ΘΚ$\frac{N%}{14}$=4ΘΚ5ΘΚ1ΘΚ2Θ§‘ρΗΟ”–ΜζΈοΒΡ Β―ι ΫΈΣC4H5ON2Θ§Ι ¥πΑΗΈΣΘΚC4H5ON2Θ°
ΒψΤά ±ΨΧβΩΦ≤ιΫœΉέΚœΘ§…φΦΑΈο÷ ΒΡΖ÷άκΓΔΧα¥ΩΓΔ”–ΜζΈοΚœ≥…ΦΑ”–ΜζΈο Β―ι ΫΒΡ»ΖΕ®Β»Θ§≤ύ÷Ί”Ύ―ß…ζΒΡΖ÷ΈωΡήΝΠΚΆ”Π”ΟΡήΝΠΒΡΩΦ≤ιΘ§ΈΣΗΏΤΒΩΦΒψΘ§ΉΔ“βΑ―Έ’Έο÷ ΒΡ–‘÷ ΒΡ“λΆ§ΦΑ”–ΜζΖ¥”ΠΈΣΫβ¥πΗΟΧβΒΡΙΊΦϋΘ§ΧβΡΩΡ―Ε»≤Μ¥σΘ°

 ΒΡΥΒΖ®÷–’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©
ΒΡΥΒΖ®÷–’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©| AΘ° | ”–7ΗωΧΦ‘≠Ή”Ω…Ρή‘ΎΆ§“Μ÷±œΏ…œ | |
| BΘ° | ÷ΜΩ…Ρή”–5ΗωΧΦ‘≠Ή”‘ΎΆ§“Μ÷±œΏ…œ | |
| CΘ° | ΉνΕύ÷ΜΩ…Ρή”–9ΗωΧΦ‘≠Ή”‘ΎΆ§“ΜΤΫΟφ…œ | |
| DΘ° | Υυ”–‘≠Ή”ΕΦΩ…Ρή‘ΎΆ§“ΜΤΫΟφ…œ |
| AΘ° | 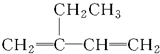 2-““Μυ-1Θ§3-ΕΓΕΰœ© 2-““Μυ-1Θ§3-ΕΓΕΰœ© | BΘ° | CH3CH2CH2CH2OH ΕΓ¥Φ | ||
| CΘ° |  ΦΉ±Ϋ ΦΉ±Ϋ | DΘ° | HOCH2CH2CH2OHΓΓ1Θ§3-Εΰ±ϊ¥Φ |
| AΘ° | SΘ®gΘ©+O2Θ®gΘ©=SO2Θ®gΘ©ΘΜΓςH=-Q kJ/molΘ°QΒΡ÷Β–Γ”Ύ297.23 kJ/mol | |
| BΘ° | SΘ®gΘ©+O2Θ®gΘ©=SO2Θ®gΘ©ΘΜΓςH=-Q kJ/molΘ°QΒΡ÷Β¥σ”Ύ297.23 kJ/mol | |
| CΘ° | 1mol SO2ΒΡΦϋΡήΉήΚΆΒ»”Ύ1molSΚΆ1molO2ΒΡΦϋΡήΚΆ | |
| DΘ° | 1mol SO2ΒΡΦϋΡήΉήΚΆ–Γ”Ύ1molSΚΆ1molO2ΒΡΦϋΡήΚΆ |
| AΘ° | ΦΉΆιΒΡ«ρΙςΡΘ–ΆΈΣ | BΘ° | ““¥ΦΒΡΖ÷Ή” ΫΈΣC2H3CH2OH | ||
| CΘ° | ΝΎΦΉΜυ±ΫΖ”ΒΡΫαΙΙΦρ ΫΈΣ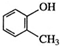 | DΘ° | Ψέ““œ©ΒΡΫαΙΙΦρ ΫΈΣCH2=CH2 |
| AΘ° | Α―Cl2Ά®»κFeCl2»ή“Κ÷– | |
| BΘ° | Α―“ΜΕΈ¥ρΡΞΙΐΒΡΟΨ¥χΖ≈»κ…ΌΝΩάδΥ°÷– | |
| CΘ° | Α―¬ΧΕΙ¥σΒΡΦΊΆΕ»κ…ΌΝΩΥ°÷– | |
| DΘ° | Α―δεΥ°ΒΈΦ”ΒΫΒμΖέKI»ή“Κ÷– |
 ΘΜ“Μ÷÷”…BΓΔCΉι≥…ΒΡΜ·ΚœΈο”κAC2ΜΞΈΣΒ»ΒγΉ”ΧεΘ§ΤδΜ·―ß ΫΈΣN2OΘ°
ΘΜ“Μ÷÷”…BΓΔCΉι≥…ΒΡΜ·ΚœΈο”κAC2ΜΞΈΣΒ»ΒγΉ”ΧεΘ§ΤδΜ·―ß ΫΈΣN2OΘ°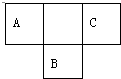 ΕΧ÷ήΤΎ‘ΣΥΊΒΡAΓΔBΓΔC‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο»γΆΦΥυ ΨΘ§“―÷ΣAΓΔBΓΔC»ΐ÷÷‘ΣΥΊΒΡ‘≠Ή”ΚΥΆβΒγΉ” ΐ÷°ΚΆΒ»”ΎBΒΡ÷ ΝΩ ΐΘ§B‘≠Ή”ΚΥΡΎ÷ Ή” ΐΚΆ÷–Ή” ΐœύΒ»Θ°Ψί¥ΥΧνΩ’ΘΚ
ΕΧ÷ήΤΎ‘ΣΥΊΒΡAΓΔBΓΔC‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο»γΆΦΥυ ΨΘ§“―÷ΣAΓΔBΓΔC»ΐ÷÷‘ΣΥΊΒΡ‘≠Ή”ΚΥΆβΒγΉ” ΐ÷°ΚΆΒ»”ΎBΒΡ÷ ΝΩ ΐΘ§B‘≠Ή”ΚΥΡΎ÷ Ή” ΐΚΆ÷–Ή” ΐœύΒ»Θ°Ψί¥ΥΧνΩ’ΘΚ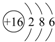
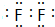 Θ§CΒΡΒΞ÷ »ή”ΎΥ°ΥυΒΟ»ή“ΚΫ–ΘΚ«βΖζΥαΘ°
Θ§CΒΡΒΞ÷ »ή”ΎΥ°ΥυΒΟ»ή“ΚΫ–ΘΚ«βΖζΥαΘ°