��Ŀ����
8����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ��������ЧӦ����Ҫ���壮B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磮���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ����1��A��B��C�ĵ�һ��������С�����˳��ΪC��O��N��
��2��B���⻯����ӵ����幹���������Σ�������ԭ�Ӳ�ȡsp3�ӻ���
��3��д��������AC2�ĵ���ʽ
 ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O����4��E�ĺ�������Ų�ʽ��1s22s22p63s23p63d54s1��ECl3�γɵ������Ļ�ѧʽΪ[Cr��NH3��4��H2O��2]Cl3��
��5��B������������Ӧ��ˮ�����Ũ��Һ��D�ĵ��ʷ�Ӧʱ������һ�ֺ���ɫ�����壬�÷�Ӧ�Ļ�ѧ����ʽ��Mg+4HNO3=Mg��NO3��2+2NO2��+2H2O��
���� A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�����֮����������ΪN��O��F�е����֣�A��B��C��ͬһ���ڵķǽ���Ԫ�أ����ڵڶ����ڣ�AC2Ϊ��������ЧӦ����Ҫ���壬������Ϊ������̼������֪AΪ̼Ԫ�ء�CΪ��Ԫ�أ�Bԭ����������̼����֮�䣬��BΪNԪ�أ�D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ����������Ϊ10����Dԭ�Ӻ��������Ϊ10+2=12����DΪMg��E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3���ݴ˽��
��� �⣺A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�����֮����������ΪN��O��F�е����֣�A��B��C��ͬһ���ڵķǽ���Ԫ�أ����ڵڶ����ڣ�AC2Ϊ��������ЧӦ����Ҫ���壬������Ϊ������̼������֪AΪ̼Ԫ�ء�CΪ��Ԫ�أ�Bԭ����������̼����֮�䣬��BΪNԪ�أ�D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ����������Ϊ10����Dԭ�Ӻ��������Ϊ10+2=12����DΪMg��E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��1��ͬ������ԭ����������һ�����ܳ��������ƣ�NԪ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ����������˼�ǣ��ʵ�һ��������С�����˳��ΪC��O��N���ʴ�Ϊ��C��O��N��
��2��B���⻯��ΪNH3�����ӵ����幹���������Σ�������Nԭ�ӳ�3���Ҽ�������1�Թ¶Ե��ӣ�������Nԭ�Ӳ�ȡsp3 �ӻ����ʴ�Ϊ�������Σ�sp3��
��3��������CO2�ĵ���ʽΪ ��һ����N��O��ɵĻ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪ N2O���ʴ�Ϊ��
��һ����N��O��ɵĻ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪ N2O���ʴ�Ϊ�� ��N2O��
��N2O��
��4��EΪCrԪ�أ�ԭ������Ϊ24��ԭ�Ӻ�����24�����ӣ���������Ų�ʽ�� 1s22s22p63s23p63d54s1��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��1s22s22p63s23p63d54s1��[Cr��NH3��4��H2O��2]Cl3��
��5��B������������Ӧ��ˮ����ΪHNO3��D�ĵ���ΪMg�����߷�Ӧ����һ�ֺ���ɫ�����壬�ú���ɫ����Ϊ�����������÷�Ӧ�Ļ�ѧ����ʽ�ǣ�Mg+4HNO3=Mg��NO3��2+2NO2��+2H2O���ʴ�Ϊ��Mg+4HNO3=Mg��NO3��2+2NO2��+2H2O��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��漰�����ܡ����ӽṹ���ӻ����������ʽ����������Ų��������ȣ��⻯��ķе������ͬ����������Ԫ���⻯��ķе�����ƶϵ�ͻ�ƿڣ��Ѷ��еȣ�

| A�� | ����Ȳ | B�� | ����ϩ | C�� | ������ϩ | D�� | ��1��3-����ϩ |
| A�� | 16��30��58��72 | B�� | 16��28��40��52 | C�� | 16��32��48��54 | D�� | 16��30��42��56 |
�����Լ���a��Ũ���� b���������� c����ʯ�� d��ˮ e��Ũ��ˮ
��������A��ϴ�� B����Һ C������
| Ҫ�ᴿ������ �����ʣ� | �Ҵ� ��ˮ�� | ��Ȳ��H2S�� | �屽 ���壩 |
| ѡ���Լ� | |||
| ���뷽�� | 1 |
��3��������ϩ�������г��õ����ϣ���ҵ����������ϩ��һ�ֹ���·�����£�
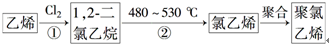
��Ӧ�ٵĻ�ѧ����ʽΪH2C=CH2+Cl2��CH2ClCH2Cl����Ӧ����Ϊ�ӳɷ�Ӧ����Ӧ�ڵķ�Ӧ����Ϊ��ȥ��Ӧ��
��4��ij�л����и�Ԫ�ص����������ǣ�̼49.5%����5.20%����16.5%����28.9%�����л����ʵ��ʽΪC4H5ON2��
| A�� | �ڶ�����Ԫ�ش�C��F���ǽ��������� | |
| B�� | ��������Ԫ�ش�Na��Cl��ԭ�Ӱ뾶������ | |
| C�� | HF��HCl��HBr��HI�����ȶ�����������ǿ | |
| D�� | LiOH��NaOH��KOH�ļ�����������ǿ |
| A�� | ���ĺ�����������Se2O3��ʽ���� | |
| B�� | ���ĺ��⻯������H2Se��ʽ���� | |
| C�� | �����⻯����ȶ��Ա������ | |
| D�� | ��������������Ӧˮ����Ļ�ѧʽ��H2SeO4 |
| A�� | һ���ǻ����� | B�� | һ���ǵ��� | ||
| C�� | �����ǻ����Ҳ�����ǵ��� | D�� | һ����ͬ�������� |