��Ŀ����
12��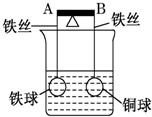 ��ͼ�ܸ�AB���˷ֱ���������ͬ������ͬ�Ŀ�������Ϳ���ͭ���ڸܸ�ʹ�䱣��ƽ�⣬һ��ʱ���С�ļ���ŨCuSO4��Һ���ش������й����⣨��������˿��Ӧ������ĸ����仯��
��ͼ�ܸ�AB���˷ֱ���������ͬ������ͬ�Ŀ�������Ϳ���ͭ���ڸܸ�ʹ�䱣��ƽ�⣬һ��ʱ���С�ļ���ŨCuSO4��Һ���ش������й����⣨��������˿��Ӧ������ĸ����仯����1�����ܸ�Ϊ��Ե�壬��A�˵ͣ���ߡ��͡�����������Ӧ�����ӷ���ʽFe+Cu2+�TFe2++Cu��
��2�����ܸ�Ϊ���壬��A�˸ߣ�ͬ�ϣ����ڴ˹�������˿���ܸˡ�С��CuSO4��Һ������ԭ��أ��缫��Ӧ�ֱ���
������Cu2++2e-�TCu��
������Fe-2e-�TFe2+��
��3�����ܸ�Ϊ���壬һ��ʱ��ͨ���ܸ˵�����Ϊ0.1NA���������������6g��
���� ��1�����ܸ�Ϊ��Ե��ʱ��ֻ����Fe������ͭ��Һ�ķ�Ӧ��
��2�����ܸ�Ϊ����ʱ�����ձ��������ŨCuSO4��Һ������Fe��Cuԭ��أ�FeΪ����������Fe-2e-�TFe2+��CuΪ����������Cu2++2e-�TCu��
��3�����ܸ�Ϊ����ʱ��FeΪ����������Fe-2e-�TFe2+��CuΪ����������Cu2++2e-�TCu����ͨ���ܸ˵�����Ϊ0.1NA�����ܽ����Ϊ0.05mol��������ͭΪ0.05mol���Դ������
��� �⣺��1�����ܸ�Ϊ��Ե��ʱ��ֻ����Fe������ͭ��Һ�ķ�Ӧ����Fe�ı��渽��Cu�����������A�˵ͣ�B�˸ߣ�������Ӧ�����ӷ���ʽΪFe+Cu2+�TFe2++Cu���ʴ�Ϊ���ͣ�Fe+Cu2+�TFe2++Cu��
��2�����ܸ�Ϊ����ʱ�����ձ��������ŨCuSO4��Һ������Fe��Cuԭ��أ�FeΪ����������Fe-2e-�TFe2+��CuΪ����������Cu2++2e-�TCu����A�˸ߣ�B�˵ͣ�
�ʴ�Ϊ���ߣ�Cu2++2e-�TCu��Fe-2e-�TFe2+��
��3�����ܸ�Ϊ����ʱ��FeΪ����������Fe-2e-�TFe2+��CuΪ����������Cu2++2e-�TCu����ͨ���ܸ˵�����Ϊ0.1NA�����ܽ����Ϊ0.05mol��0.05��56=2.8g��������ͭΪ0.05mol��0.05��64=3.2g�����������������2.8+3.2=6 g���ʴ�Ϊ��6��
���� ���⿼��ԭ��ؼ���ѧ��Ӧ����ȷ�ܸ��Ƿ缰�����ķ�Ӧ�ǽ����Ĺؼ���ѧ�������Ըܸ�Ϊ��Ե��ʱ���������Ŀ�Ѷ��еȣ�

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д�| A�� | 80% | B�� | 75% | C�� | 60% | D�� | 40% |
 ���ݻ�һ�����ܱ������У����淴Ӧ��A2 ��g��+B2��g��?xC��g�� ��������ͼ����ʾ��ϵ���ɴ��ƶϣ���ͼ���˵����ȷ���ǣ�������
���ݻ�һ�����ܱ������У����淴Ӧ��A2 ��g��+B2��g��?xC��g�� ��������ͼ����ʾ��ϵ���ɴ��ƶϣ���ͼ���˵����ȷ���ǣ�������| A�� | P3��P4��Y���ʾ��������ƽ��Ħ������ | |
| B�� | P3��P4��Y���ʾ���������ܶ� | |
| C�� | P3��P4��Y���ʾA2��Ũ�� | |
| D�� | P3��P4��Y���ʾA2��ת���� |
| A�� | pH=0����Һ�У�ClO-��Cu2+��SO42-��K+ | |
| B�� | ʹpH��ֽ���ɫ����Һ�У�Fe2+��I-��NO3-��Cl- | |
| C�� | �ܹ��ͽ���þ��Ӧ�ų��������Һ�У�Na+��H+��SO42-��Cl- | |
| D�� | �����£�ˮ�������c��H+����c��OH-���˻�Ϊ10-28����Һ�У�K+��Na+��HS-��Ca2+ |
| Ԫ�ر�� | A | B | C | D | E | F | G | H | I | J |
| ԭ�Ӱ뾶��10-10m�� | 1.02 | 2.27 | 0.74 | 1.43 | 0.77 | 1.10 | 0.99 | 1.86 | 0.75 | 1.17 |
| ������� | +6 | +1 | �� | +3 | +4 | +5 | +7 | +1 | +5 | +4 |
| ����� | -2 | �� | -2 | �� | -4 | -3 | -1 | �� | -3 | -4 |
��1������10��Ԫ���е�һ��������С����K����Ԫ�ط��ţ���
��2��д�������йط�Ӧ�Ļ�ѧ����ʽ��E�ĵ�����IԪ�ص�����������Ӧ��ˮ���ﷴӦ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��
��3����Ԫ��Bԭ��������11��Ԫ�صĻ�̬ԭ�ӵ����Ų�ʽ��[Ar]3d104s2��
��4��C��I��Ƚϣ��ǽ����Խ�������N����Ԫ�ط��ţ���������֤��Ľ��۵��������е�ab�����ţ���
a����̬�⻯����ȶ���
b����Ԫ�صĵ縺��
c�������������
d������ϼۣ�
| ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | C | N | O | Ne | ||||
| 3 | Na | Mg | Al | Si | S | Cl |
��2����ѧ��������õ�Ԫ����Ne����Ԫ�ط��ţ���
��3��þ��ԭ�ӽṹʾ��ͼΪ
 ��
����4��C��N��ȣ�ԭ�Ӱ뾶��С����N��
��5������������Ӧ��ˮ����������ǿ����HClO4������ǿ����NaOH���ѧʽ����
��6���������ư뵼����ϵ�Ԫ���ǹ裮
��7��H2S��HCl��ȣ����ȶ��Խ�ǿ����HCl��
��8�����һ���������ʹƷ����Һ��ɫ��д����������Ļ�ѧʽSO2��������Ԫ�غ���Ԫ�ص�������m��S����m��O��=1��1��
��9�������½���������ˮ���ҷ�Ӧ����д����Ӧ�Ļ�ѧ����ʽ��2Na+2H2O=2NaOH+H2����
| A�� | Һ�ȡ����� | B�� | CH2=CH-CH3 CH3-CH2-CH3 | ||
| C�� | N2��CO | D�� | 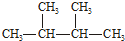 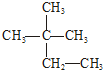 |