��Ŀ����
����Ŀ����1������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬�������⡣����0.4molҺ̬�º�0.8mol H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�257.7kJ������(�൱��25����101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ ������֪H2O(l)��H2O(g) ��H��+44kJ/mol����16gҺ̬����������ⷴӦ����Һ̬ˮʱ�ų��������� kJ��
��2���ֱ�ȡ40 mL 0.50 mol/L������ 0.55 mol/L NaOH��Һ�����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
�������������������Һ���ܶȶ���1 g/cm3����֪�кͺ�������Һ�ı�����c = 4.18 J/(g����)��ʵ��ʱ����������������� ��
A����Ӧǰ������Һ���¶�
B����Ӧǰ������Һ������
C����Ӧǰ����������Һ���¶�
D����Ӧǰ����������Һ������
E����Ӧ������Һ������¶�
F����Ӧ������Һ������
ijѧ��ʵ���¼�������£�
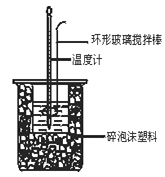
ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
���� | �������� | �����Һ | |
1 | 20.0 | 20.1 | 23.2 |
2 | 20.2 | 20.4 | 23.4 |
3 | 20.5 | 20.6 | 23.6 |
���ݸ�ѧ����ʵ�����ݼ��㣬��ʵ���õ��к���Ϊ��H = ____________��
�ٶ���ѧ���IJ�����ȫͬ�ϣ�ʵ���и���100 mL 0.50 mol/L�����100 mL 0.55 mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� �������������������������������к��� ��������������������������
���𰸡���1��N2H4(l)+2H2O2(l) == N2(g) +4H2O(g) ��H����644.25 k/mol 410.125
��2��A��C��E ��H = -51.8 kJ/mol ����� ���
��������
�����������1����֪0.4molҺ̬�º�0.8mol˫��ˮ��Ӧ���ɵ�����ˮ����ʱ�ų�257.7kJ���������º�˫��ˮ��Ӧ���Ȼ�ѧ����ʽ��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g�� ��H����644.25 k/mol��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H����644.25 k/mol����H2O��l����H2O��g������H��+44KJ/mol�����ݸ�˹������-����4�õ���N2H4��l��+2H2O2��l���TN2��g��+4H2O��L����H��-820.25kJ/mol����ѧ����ʽ��32gȫ����Ӧ����820.25kJ��16gҺ̬��������˫��ˮ��Ӧ���ɵ�����Һ̬ˮʱ���ų���������410.125kJ��
��2������Q��cm��T��֪���ⶨ�к�����Ҫ�ⶨ������Ϊ��A����Ӧǰ������Һ���¶ȡ�B����Ӧǰ������Һ��������E����Ӧ������Һ������¶ȣ���ѡACE��
����1��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.05������Ӧ���¶�Ϊ��23.2������Ӧǰ���¶Ȳ�Ϊ��3.15������2��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.3������Ӧǰ���¶Ȳ�Ϊ��3.1������3��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.55������Ӧǰ���¶Ȳ�Ϊ��3.05����40mL��0.50mol/L������40mL��0.55mol/L����������Һ��������m��80mL��1g/mL��80g��c��4.18J/��g���������빫ʽQ��cm��T������0.02mol��ˮ�ų�����Q��4.18J/��g������80g����3.15��+3.1��+3.05�� ��/3��1.036kJ��������0.02mol��ˮ�ų�����Ϊ��1.036kJ����������1mol��ˮ�ų�����Ϊ��1.036kJ��1mol/0.02mol����51.8kJ/mol������ʵ���õ��к�����H=-51.8kJ/mol��
����Ӧ�ų����������������Լ�������Ķ����йأ�����100mL 0.50mol/L�����100mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������ӣ����ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�������100mL 0.50mol/L�����100mL 0.55mol/L NaOH��Һ��������ʵ�飬����к�����ֵ�����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�