��Ŀ����
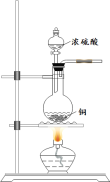
����Ŀ��ijͬѧ��������ͭ����(![]() )�ᾧˮ�����IJⶨʵ�顣���������գ�
)�ᾧˮ�����IJⶨʵ�顣���������գ�
��ʵ�鲽�裩��
(1)��__________(����������)ȷ������������������
(2)�ڴ������м���һ����������ͭ���壬�����ء�
(3)��ʢ������ͭ����Ĵ������������������������ȣ�ֱ����ɫ��ȫ��ף�Ȼ�����������_______(����������)����ȴ�����£������ء�
(4)�ظ�����ʵ����к��ز�������Ŀ����_______________��ֱ�����γ������������______�ˡ�
(5)�����Ǹ�ѧ��ʵ���һ�����ݣ�����ɼ��㣺
��������(��) | �����뾧�������(��) | ���غ�������������� |
13.721 | 24.692 | 20.631 |
![]() ______��(��ȷ��0.01)ʵ����������_________(����С����һλ)��
______��(��ȷ��0.01)ʵ����������_________(����С����һλ)��
(6)���ʵ���в�������ԭ�������_______
a. ����ͭ�����к��в��ӷ������� b. �ڼ��ȹ����з����к�ɫ��������
c. ����ʱ�о���ɽ����� d. ����ʧˮ��¶���ڿ�������ȴ
���𰸡�������ƽ ������ ȷ���ᾧˮʧȥ��ȫ 0.001 5.22 4.4% bc
��������
��1�����ݾ�ȷ��ѡ�����������
��3���ڼ��Ⱥ���ȴʱ��Ϊ�˷�ֹ����ͭ��ˮ��Ӧ������ͭ���ڸ������н�����ȴ���Ӷ��õ��������������ˮ����ͭ��
��4��ȡ����ƽ��ֵ������������Ϊȷ������ͭ������ȫʧȥ�ᾧˮ���������ȴ�����ټ�����ȴ������ֱ���������γ����IJ����0.001gΪֹ������Ϊ��ȷ����Ʒ�нᾧˮ�Ƿ��Ѿ���ȫ����ȥ��
��6���ڲⶨ����������Ʒ�к��м��Ȼӷ������ʻ�ʵ��ǰ������Ʒ���泱ʪ��������ɲ������ƫ�ߣ�
��1��������ƽ��ȷ��Ϊ0.1g��������ƽ�ɾ�ȷ��0.001g���������ݿ�֪��Ӧѡ�������ƽ��
�ʴ�Ϊ��������ƽ��
��3��������ˮ����ͭ���ˮ����Ӧ���ڸ��������ܷ���ȴ��
�ʴ�Ϊ����������
��4��Ϊȷ������ͭ������ȫʧȥ�ᾧˮ���������ȴ�����ټ�����ȴ������ֱ���������γ����IJ����0.001gΪֹ������Ϊ��ȷ����Ʒ�нᾧˮ�Ƿ��Ѿ���ȫ����ȥ����֤�ᾧˮȫ��ʧȥ��
�ʴ�Ϊ��ȷ���ᾧˮʧȥ��ȫ ��0.001��
��5�� ![]() = CuSO4+ xH20
= CuSO4+ xH20
160+18x 160
(24.692-13.721) (20.631-13.721)
![]() =
=![]()
��ã�x=5.22
ʵ��������=![]() ��100%=4.4%��
��100%=4.4%��
�ʴ�Ϊ��5.22��4.4%��
��6��a.������Ʒ�к��м��Ȳ��ӷ������ʻᵼ�²ⶨ��ˮ������ƫС����a����
b.�ڼ��ȹ����з����к�ɫ�������ɣ�˵������ͭ�ֽ⣬ʹ����ͭ������ƫС���ᾧˮ������ƫ���½��ƫ��b��ȷ��
c.���ȹ��������������彦��������ˮ�������ⶨ���ƫ��c��ȷ��
d.���Ⱥ�����δ�������������ȴ���ᵼ�²ⶨ������ͭ������ƫ�ⶨ��ˮ������ƫС����d����
�ʴ�Ϊ��bc��

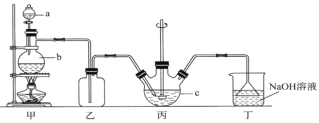
����Ŀ���Ȼ�隣������̿��Ʊ��ߴ���̼���̵Ĺ����������£�
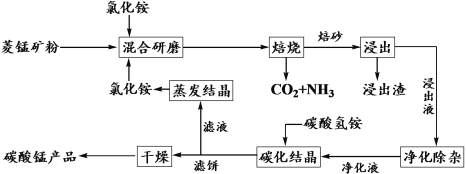
��֪�������̿�ʯ��Ҫ�ɷ���MnCO3������������Fe��Al��Ca��Mg��Ԫ�أ�
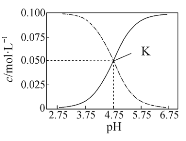
����ؽ�������[c(Mn+)=0.1 mol��L1]�γ��������������pH���£�
�������� | Al3+ | Fe3+ | Fe2+ | Ca2+ | Mn2+ | Mg2+ |
��ʼ������pH | 3.8 | 1.5 | 6.3 | 10.6 | 8.8 | 9.6 |
������ȫ��pH | 5.2 | 2.8 | 8.3 | 12.6 | 10.8 | 11.6 |
�ش��������⣺
(1)�����ա�ʱ��Ҫ��Ӧ�Ļ�ѧ����ʽΪ__________________��
(2)��������ͼ1��ͼ2��ͼ3���Ȼ�隣�����þ�����������ǣ�_________________��

(3)����Һ���������ӡ��������£����ȼ���MnO2��Fe2+����ΪFe3+����Ӧ�����ӷ���ʽΪ________________________��Ȼ�������ҺpHʹFe3+��Al3+������ȫ��
(4)̼���ᾧʱ��������Ӧ�����ӷ���ʽΪ___________��̼���ᾧ�����в�����̼�����Һ����̼�������Һ���ܵ�ԭ����___________________��
(5)��������ѭ�����õĹ�̬������____________��