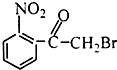
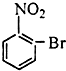
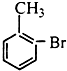
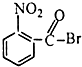��Ŀ����
8��ij�ᾧˮ����Ļ�ѧʽΪR•nH2O������Է�������ΪM��25��ʱ����a g�þ�������b gˮ��ǡ�ÿ��γ�V mL������Һ�����й�ϵ��ȷ���ǣ�������| A�� | ������Һ�����ʵ���Ũ��c=$\frac{1000a��M-18n��}{MV}$mol/L | |
| B�� | ������Һ�����ʵ���������w=$\frac{a��M-18n��}{M��a+b��}$% | |
| C�� | 25��ʱR���ܽ��S=$\frac{100a��M-18n��}{18na+Mb}$g | |
| D�� | ������Һ���ܶȦ�=$\frac{a��M-18n��}{a+b}$g/L |
���� A������n=$\frac{m}{M}$����R•nH2O�����ʵ�������n��R��=n��R•nH2O��������c=$\frac{n}{V}$����ñ�����Һ�����ʵ���Ũ�ȣ�
B������w�����ʣ�=$\frac{m�����ʣ�}{m����Һ��}$��100%����ñ�����Һ����������
C�����ݻ�ѧʽ����ᾧˮ��������R������������������Һ��ˮ��������������100g�ܽ�R��������Ϊ�ܽ�ȣ�
D����Һ������Ϊ��a+b��g�����ݦ�=$\frac{m}{V}$������Һ�ܶȣ�������c=$\frac{1000�Ѧ�}{M}$���й�ʾ���μ��㣮
��� �⣺A��n��R��=n��R•nH2O��=$\frac{a}{M}$mol���ñ�����Һ�����ʵ���Ũ��$\frac{\frac{a}{M}mol}{V��1{0}^{-3}L}$=$\frac{1000a}{MV}$mol/L����A����
B������w�����ʣ�=$\frac{m�����ʣ�}{m����Һ��}$��100%����֪�ñ�����Һ��������Ϊ$\frac{\frac{M-18n}{M}��ag}{ag+bg}$��100%=$\frac{100a��M-18n��}{M��a+b��}$%��
��B����
C��R������Ϊ$\frac{M-18n}{M}$��ag���ᾧˮ������Ϊ$\frac{18n}{M}$��ag����100g��S=��$\frac{18n}{M}$��ag+bg����$\frac{M-18n}{M}$��ag�����S=$\frac{100a��M-18n��}{18na+bM}$g����C��ȷ��
D����Һ������Ϊ��a+b��g�����ݦ�=$\frac{m}{V}$��֪����Һ�ܶ�Ϊ$\frac{��a+b��g}{VmL}$=$\frac{a+b}{V}$g/mL������c=$\frac{1000�Ѧ�}{M}$��֪���ܶȦ�=$\frac{cM}{1000��}$=$\frac{1000a}{MV}$����M-18n���£�1000��$\frac{100a��M-18n��}{M��a+b��}$%��g/mL=$\frac{a+b}{V}$g/mL����D����
��ѡC��
���� ���⿼����ҺŨ�ȼ��㣬�漰���ʵ���Ũ�ȡ������������ܽ�ȣ�������ĸ�ͼ��㣬Ϊ�״���Ŀ��ע��Ի���֪ʶ���������գ�

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д� Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�| A�� | 9.1% | B�� | 10.5% | C�� | 10% | D�� | 5% |
| A�� | ����������Һ�м�������������Һ��H++OH-=H2O | |
| B�� | ����ͭ��ϡ���CuO+2H+=Cu2++H2O | |
| C�� | ������NaHCO3��������NaOH��Һ��HCO3-+OH-=CO32-+H2O | |
| D�� | ������NaHCO3��Һ��������Ca��OH��2��Һ��2HCO3-+Ca2++2OH-=CaCO3��+CO32-+2H2O |
| A�� | �����˵�վй©�ķ�����������131I��127I��Ϊͬλ�أ���ѧ���ʼ�����ͬ | |
| B�� | ���⻯ѧ�����������������ꡱ���γɶ��뵪���������й� | |
| C�� | �ɷ�����ɵ������в�һ�����ڹ��ۼ� | |
| D�� | ����״̬�ܵ��������һ�������ӻ����� |
| A�� | ��a=0.5����Cu2+������ǡ��ȫ������ʱ������������ֻ������ | |
| B�� | ��a=0.5����Cu2+������ǡ��ȫ������ʱ����������������ʵ���Ϊ0.1mol | |
| C�� | ��a=0.2����Cl-������ǡ��ȫ���ݳ�ʱ������0.08mol��NaOH | |
| D�� | �����һ��ʱ���������������������������ʵ���ǡ�þ�Ϊ0.16mol����a=0.2 |
| ��������ĩ״�� | ���Ũ�ȼ���� | ��Ӧ�¶� | |
| A | Mg 0.1g | 18.4mol•L-1����10mL | 80�� |
| B | Mg 0.1g | 1.5mol•L-1����10mL | 60�� |
| C | Fe 0.1g | 1mol•L-1���� 10mL | 60�� |
| D | Mg 0.1g | 1mol•L-1����10mL | 60�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ɫ��Һ�п��ܴ�������Al3+��NH4+��Cl?��[Al��OH��4]? | |
| B�� | ������Һ�п��ܴ�������Na+��ClO?��SO42?��I? | |
| C�� | ������Һ�п��ܴ�������Fe3+��K+��Cl?��SCN? | |
| D�� | ���������ܷų���������Һ���ܴ�������K+��Ba2+��Cl-��Br- |