��Ŀ����
����Ŀ����.ͼ1��ʵ�������Ҵ���Ũ��������ϩ�ķ���װ�ã�ͼ2����ϩ����ʵ��װ�ã���ش�
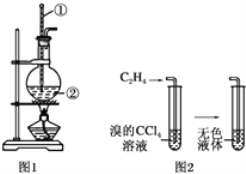
(1)ͼ1�������١��ڵ����Ʒֱ�Ϊ________��________��
(2)��д��ʵ��������ϩ�Ļ�ѧ����ʽ��________��
(3)��������Ȼ�̼��Һ��ͨ����ϩ����Һ����ɫ�ܿ���ȥ���÷�Ӧ����________(�Ӧ����)��
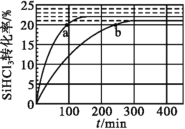
��.ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ���������ش�

(1)ͼ�Т١��ڡ��ۡ���װ��ʢ�ŵ��Լ��������е�(�뽫�����й��Լ�����������Ӧװ���ڣ����ظ�ѡ��)����_____��_____��______��________
A.Ʒ����Һ B.����������Һ C.Ũ���� D.���Ը��������Һ
(2)��˵����������������ڵ�������_______��
(3)ʹ��װ�âڵ�Ŀ����________��
(4)ʹ��װ�â۵�Ŀ����________��
(5)��֤������ϩ��������_______��
���𰸡��¶ȼ� Բ����ƿ CH3CH2OH![]() CH2=CH2��+H2O �ӳ� A B A D ����Ʒ����Һ��ɫ ���ն����������� ������������Ƿ���� ����Ʒ�첻��ɫ�����и��������ɫ
CH2=CH2��+H2O �ӳ� A B A D ����Ʒ����Һ��ɫ ���ն����������� ������������Ƿ���� ����Ʒ�첻��ɫ�����и��������ɫ
��������
II��Ϊ��֤���������������ϩ�Ͷ��������Ƚ��������ͨ��Ʒ����Һ���Դ˼���SO2����ͨ��NaOH��Һ����SO2���ų����Լ�����ϩ�ĸ��ţ�����ǰ�ٴ���Ʒ����Һ����SO2�Ƿ�����������ͨ�����Ը��������Һ�м�����ϩ��
I��(1) ͼ1�����������¶ȼƣ���������Բ����ƿ��
(2)ʵ�����Ʊ���ϩ�Ǽ����Ҵ���Ũ����Ļ������170���Ƶã���Ӧ����ʽΪ��CH3CH2OH![]() CH2=CH2��+H2O��
CH2=CH2��+H2O��
(3)��������Ȼ�̼��Һ��ͨ����ϩ����ϩ���巢���ӳɷ�Ӧ����1,2-�������飬��Ӧ����Ϊ�ӳɷ�Ӧ��
II��(1)����SO2����ϩ����ʹ��ˮ�����Ը��������Һ��ɫ����������Ҫ����SO2������Ʒ����Һ��Ϊ��ֹ������ϩ�ļ��飬����Ҫ��ȥSO2����������������Һ������Ҫ�ٴ�ͨ��Ʒ����Һ������SO2�Ƿ�������������ͨ�뵽���Ը��������Һ��������ϩ���ʴ�Ϊ��A��B��A��D��
(2)��˵����������������ڵ������ǣ�����Ʒ����Һ��ɫ��
(3)װ�â���װ��NaOH��Ŀ���ǣ����ն����������壬���������ϩ�ļ��飻
(4)װ�â�ͨ���۲�Ʒ����Һ����ɫ��ȷ��SO2�ѳ��ɾ�����Ϊ��������������Ƿ������
(5)װ�â�ͨ�����������Һ��ɫ��������ϩ������֤������ϩ�������ǣ�����Ʒ�첻��ɫ�����и��������ɫ��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�