��Ŀ����
����Ŀ���ɺϳ����Ʊ������ѣ��漰���·�Ӧ��
��i��2CH3OH(g)![]() C2H4(g)+2H2O(g) ��H1
C2H4(g)+2H2O(g) ��H1
��ii��2CH3OH(g)![]() CH3OCH3(g)+H2O(g) ��H2
CH3OCH3(g)+H2O(g) ��H2
�����仯��ͼ��ʾ��
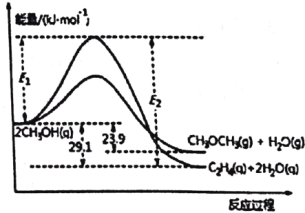
����˵����ȷ����
A. ��H1����H2
B. ��Ӧ��ii��Ϊ���ȷ�Ӧ
C. C2H4(g)+H2O(g)![]() CH3OCH3(g) ��H = -5.2 kJ��mol��1
CH3OCH3(g) ��H = -5.2 kJ��mol��1
D. ���������м����������E2-E1����С
���𰸡�A
��������
A. ����ͼ�����ݿ�֪����Ӧ�����з���Խ�࣬���Ӧ�ķ�Ӧ����HԽС��
B. ��Ӧ����������������������������
C. ���ݸ�˹���ɷ�������
D. �������Խ��ͷ�Ӧ�Ļ�ܣ������ı䷴Ӧ�ȣ�
A. ����ͼ�����ݿ�֪����H1 = -29.1 kJ��mol��1����H2 = -23.9 kJ��mol��1������H1����H2����A����ȷ��
B. ��ͼ�����ݿɿ�������H2��0������Ӧ��ii��Ϊ���ȷ�Ӧ����B�����
C. ���ݸ�˹���ɿ�֪����Ӧ��ii��-��Ӧ��i���ɵ÷�ӦC2H4(g)+H2O(g)![]() CH3OCH3(g)������H = ��H2-��H1 = -23.9 kJ��mol��1-��-29.1 kJ��mol��1��= +5.2 kJ��mol��1����C�����
CH3OCH3(g)������H = ��H2-��H1 = -23.9 kJ��mol��1-��-29.1 kJ��mol��1��= +5.2 kJ��mol��1����C�����
D. ���������м�������������˷�Ӧ�Ļ�ܣ�������ı䷴Ӧ�ʱ䣬��E2-E1�IJ�ֵ���䣬��D�����
��ѡA��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ijʵ��С���ô�п������������ˮ�Ҳ����ᷴӦ����100gϡ���ᷴӦʱ��ʵ���������±���ʾ��
ʵ����� | ��п����/g | ϡ��������/g | ������������/g | ��Ӧ��ʣ���������/g |
ʵ��1 | 8 | 100 | 0.2 | 1.5 |
ʵ��2 | 16 | 100 | 0.4 | 3 |
ʵ��3 | 30 | 100 | 0.6 | 10.5 |
��1��ʵ����ϡ���ᷴӦ�����______����ʵ����ţ���
��2������ϡ�����������������______��




