��Ŀ����
����Ŀ���Թ�ҵ��ˮ��������ˮ���д����Ƿ�ֹˮ����Ⱦ������ˮ�ʵ���Ҫ��ʩ֮һ��
��1����Ƴ��ķ�ˮ�к��е�CN�о綾����Ҫ���������ŷš�������CN��ˮ�ķ���֮һ��������������£�CN������������HCO3-��ͬʱ����NH3���÷�Ӧ�����ӷ���ʽΪ__��
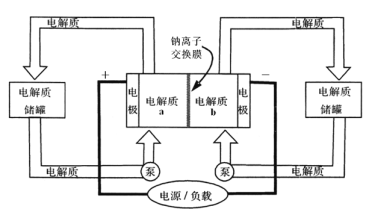
��2��������������������������Һ��ͬʱ�õ������ԭ����ͼ��ʾ(ͼ����HA����ʾ������ӣ�A��ʾ���������)��
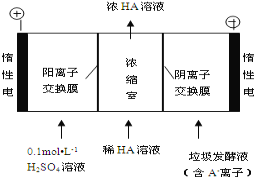
�������ĵ缫��ӦʽΪ___��
�ڼ���Ũ�����еõ�Ũ�����ԭ����__��
�۵������У���ȡһ���Ĵ�ʩ�ɿ��������ҵ�pHԼΪ6��8����ʱ����Ũ���ҵ�OH�ɺ��Բ��ơ�400mL10g/L������Һͨ��һ��ʱ���Ũ������Ϊ145g/L(��Һ����仯���Բ���)�������ϲ�����H2�ڱ�״���µ����ԼΪ__L(��ʾ�������Ħ������Ϊ90g/mol)��
���𰸡�4H2O+2CN+O2=2HCO3-+2NH3�� 2H2O-4e=4H++O2�� ����H2O�ŵ磬c(H+)����H+������ͨ�������ӽ���Ĥ����Ũ���ң�A������ͨ�������ӽ���Ĥ����Ũ���ң�H++A=HA������Ũ������ 6.72
��������
��1��CN��ˮ�ķ���֮һ��������������£�CN������������HCO3��ͬʱ����NH3����������غ���д��ѧ����ʽ��
��2�����ݵ��صĹ���ԭ���������������������������ӷ���ʧ���ӵ�������Ӧ���жϣ����ݵ��صĹ���ԭ�����ڵ��ص���������OH�ŵ磬����H+������ͨ�������ӽ���Ĥ����Ũ���ң��ٸ��ݲ������������㡣
��1�����������Ϣ֪����Ӧ��ΪCN��O2��ˮ��������ΪCN��HCO3-�������ݵ�ʧ�����غ���ƽ��Ӧ�õ����ӷ���ʽΪ��4H2O+2CN+O2=2HCO3-+2NH3�����ʴ�Ϊ�� 4H2O+2CN+O2=2HCO3-+2NH3����
��2����������ˮʧȥ���ӣ�����������Ӧ��������������ӦʽΪ��2H2O-4e=4H++O2����
���������Ϸ����缫��Ӧ��2H2O-4e=4H++O2���������Ϸ����缫��Ӧ��2H++2e-=H2�������ݵ缫��Ӧʽ�����й�ϵ��HA~H+~![]() H2���ݲ�ֵ���������Ũ�ȱ仯���ǣ�
H2���ݲ�ֵ���������Ũ�ȱ仯���ǣ�![]() �� ������HA�����ʵ�����1.5mol/L��0.4L=0.6mol������������0.3mol����0.3mol��22.4L/mol=6.72L���ʴ�Ϊ��2H2O-4e=4H++O2��������H2O�ŵ磬c(H+)����H+������ͨ�������ӽ���Ĥ����Ũ���ң�A������ͨ�������ӽ���Ĥ����Ũ���ң�H++A=HA������Ũ������ 6.72��
�� ������HA�����ʵ�����1.5mol/L��0.4L=0.6mol������������0.3mol����0.3mol��22.4L/mol=6.72L���ʴ�Ϊ��2H2O-4e=4H++O2��������H2O�ŵ磬c(H+)����H+������ͨ�������ӽ���Ĥ����Ũ���ң�A������ͨ�������ӽ���Ĥ����Ũ���ң�H++A=HA������Ũ������ 6.72��

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�