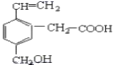
��Ŀ����
����Ŀ����1��25��ʱ��Ũ��Ϊ0.1 mol��L��1��6����Һ����HCl�� ��CH3OOH�� ��Ba(OH)2����Na2CO3����KCl����NH4Cl��ҺpH��С�����˳��Ϊ__________________(��д���)��
��2��25��ʱ������ĵ��볣��Ka=1.7��10-5mol/L������¶���CH3COONa��ˮ��ƽ�ⳣ��Kh=_________mol ��L-1��������С�����һλ����
��3��25��ʱ��pH��3�Ĵ����pH��11������������Һ�������Ϻ���Һ��_________���������������������������������� ����д����Һ������Ũ�ȼ��һ����ʽ��________________________________��
��4��25��ʱ����m mol/L�Ĵ����n mol/L������������Һ�������Ϻ���Һ��pH��7������Һ��c(CH3COO��) + c(CH3COOH)=______��m��n�Ĵ�С��ϵ�ǣ�_____����� ��������������<������
��5��25��ʱ����������������ʵ���Ũ�ȵĴ����백ˮ��Ϻ���Һ��pH��7 ����NH3��H2O�ĵ��볣��Ka=______________��
���𰸡��٢ڢޢݢܢ� 5.9��10-10 ���� c(Na��) + c(H��) = c(CH3COO��) + c(OH��) ![]() �� .7��10-5mol/L
�� .7��10-5mol/L
��������
��1����HCl��һԪǿ�ᣬ ��CH3OOH��һԪ���ᣬ ��Ba(OH)2�Ƕ�Ԫǿ���Na2CO3��ǿ�������Σ���KCl��ǿ��ǿ���Σ���NH4Cl��ǿ�������Ρ����ԣ�ǿ������������ǿ�������Σ����ԣ���Ĵ���ǿ�������εġ������⼸����ҺpH��С�����˳��Ϊ�٢ڢޢݢܢ�.
��2��KHAc![]() CH3COO-+H+,
CH3COO-+H+,![]() �����¶���CH3COONa��ˮ��ƽ��ΪCH3COO-+H2O
�����¶���CH3COONa��ˮ��ƽ��ΪCH3COO-+H2O![]() CH3COOH+OH-��ˮ��ƽ�ⳣ��
CH3COOH+OH-��ˮ��ƽ�ⳣ��![]()
![]()
![]() ������
������![]() ��
��
��3��25��ʱ��pH��3�Ĵ��ᣬc(H+)=10-3mol/L�� pH��11������������Һ,c(H+)=10-11mol/L����c(OH-)=Kw��c(H+)=10-14��10-11=10-3mol/L��������Һ�е�����Ũ����ȡ����������Ϻ���IJ���ǡ����ȫ�к͡������ڴ���Ϊ���ᣬ���д���δ����Ĵ�����Ӵ��ڣ�������������H+��CH3COO-��������Һ�����ԡ�����Һ�д��ڵ���غ㡣c(Na��) + c(H��) = c(CH3COO��) + c(OH��)��
��4��������ҺΪ�������ϣ�������Һ��c(CH3COO��) + c(CH3COOH)=![]() mol/L����Ϊ�������ᣬ����ǿ��������ʵ�����ϣ�ǡ������CH3COONa����Һ����CH3COO-��ˮ���Լ��ԡ�Ϊ��ʹ��Һ�����ԣ������������һЩ�����������������ˮ��ļ��ԡ�����m��n�Ĵ�С��ϵ��m��n��
mol/L����Ϊ�������ᣬ����ǿ��������ʵ�����ϣ�ǡ������CH3COONa����Һ����CH3COO-��ˮ���Լ��ԡ�Ϊ��ʹ��Һ�����ԣ������������һЩ�����������������ˮ��ļ��ԡ�����m��n�Ĵ�С��ϵ��m��n��
��5��25��ʱ����������������ʵ���Ũ�ȵĴ����백ˮ��Ϻ���Һ��pH��7 ��˵��������һˮ�ϰ���ǿ���̶���ͬ��Ҳ���ǵ���̶���ȡ����ڴ���ĵ���ƽ�ⳣ��ΪKa=1.7��10-5mol/L������NH3��H2O�ĵ��볣��Ka=1.7��10-5mol/L��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��CO��SO2�Ǵ�����Ⱦ���壬���û�ѧ��Ӧ��������Ⱦ����Ҫ������
��.�״����Բ���Ͳ������ʯ��ȼ�ϣ�������Դ���ţ�����CO���Ժϳɼ״���CO+2H2![]() CH3OH(g)��һ�������£����ݻ�ΪVL���ܱ������г���a mol CO��2a mol H2�ϳɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g)��һ�������£����ݻ�ΪVL���ܱ������г���a mol CO��2a mol H2�ϳɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

(1)������˵����Ӧ�ﵽƽ��״̬����_______(�����)��
��v��(CO)=2v��(H2)
��c(CO)=c(CH3OH)
�ۻ�������ƽ����Է�����������
�ܵ�λʱ��������2n mol H2��ͬʱ����n mol CH3OH
(2)�÷�Ӧ��A���ƽ�ⳣ��K_________(��a��V��ʾ)��
(3)д����������v(CO)�������COת���ʵ�һ���ʩ��________
��.ijѧϰС����SO2Ϊԭ�ϣ����õ绯ѧ������ȡ���ᡣ
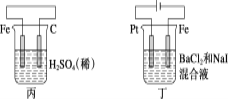
(4)ԭ���ԭ������С����Ƶ�ԭ��ʾ��ͼ(��ͼ1��ʾ)��д���õ�������ĵ缫��Ӧʽ_______��

(5)���ԭ������С����Na2SO3��Һ�������SO2�õ�NaHSO3��Һ��Ȼ�������Һ�Ƶ�������(ԭ����ͼ2��ʾ)��д����ʼ���ʱ�����ĵ缫��Ӧʽ_______��
��.���������(Na2S2O3)�׳ƴ��մ����Ź㷺����;����SO2����Na2S2O3��ijС��ͬѧ�Ʊ���Ԥ�Ⲣ̽����������Ƶ�����(��Ӧ������Һ�н���)��
Ԥ�� | ʵ����� | ʵ������ | |
̽��1 | Na2S2O3��Һ�ʼ��� | ��pH��ֽ���ڲ���Ƭ�ϣ��ò�����պȡ��Һ������ֽ�� | pH=8 |
̽��2 | Na2S2O3���л�ԭ�� | ��������ˮ�еμ�Na2S2O3��Һ | ����ɫ��ɫ��dz��������ȥ |
(6)����SO2���Ʊ�Na2S2O3������������_________��
(7)�����ӷ���ʽ��ʾNa2S2O3��Һ���м��Ե�ԭ��_________��
(8)̽��2��Ӧ�����ӷ���ʽΪ_________��