��Ŀ����
10��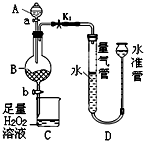 ��ҵ�����л���������ķ���м���ɽ��䡰���Ϊ�����Ƴɻ���ԭ���Ȼ�����ʵ������������ͼ��ʾװ��̽���ɷ���м�Ʊ�FeCl3•6H2O�����ԭ�����ⶨ��м�������ʵ��������������ʲ�����ˮ�Ҳ����ᷴӦ����
��ҵ�����л���������ķ���м���ɽ��䡰���Ϊ�����Ƴɻ���ԭ���Ȼ�����ʵ������������ͼ��ʾװ��̽���ɷ���м�Ʊ�FeCl3•6H2O�����ԭ�����ⶨ��м�������ʵ��������������ʲ�����ˮ�Ҳ����ᷴӦ������1��װ��A������Ϊ��Һ©����
��2�������װ�������Եľ���������£�
�ٹرջ���a�ͻ���b�����ɼ�K1������ˮ���м�ˮ��ʹˮ��Һ�����������Һ�棻��һ��ʱ���ˮ����Һ�������������Һ�棬��Һ�����ȶ���ȡm g����м����Bװ���У���A�м��������������������в�����
���ɼ�K1���رջ���b������a�������μ����ᣮ
��װ��D��������һ��Һ�治���½�ʱ���رյ��ɼ�K1������b����A����Һ��ȫ�����ձ���رջ���a��b��
���ձ��е���Һ����һϵ�в�����õ�FeCl3•6H2O���壮
��ش�
��3�������ӷ���ʽ��ʾ�ձ���������H2O2��Һ�����ã�H2O2+2Fe2++2H+=2Fe3++2H2O��
��4��ʵ������������ܺ�ˮ����Һ��߶�����ͼ��ʾ��Ϊ��ʹ����Һ����ƽ��Ӧ��ˮ�����ƣ�����ơ������ơ�����
��5����FeCl3��Һ�Ƶ�FeCl3•6H2O����IJ��������в���Ҫʹ�õ�������de����ѡ����ţ���
a�������� b���ձ� c���ƾ��� d����Һ©�� e������ f�������� g��©��
��6��ʵ����������������ڹ��ռ���VmL���壨�ѻ���ɱ�״��������˷���м�������ʵ���������Ϊ$\frac{2.5V��1{0}^{-3}}{m}$��100%��
���� ��1������װ��ͼ������
��2������װ�õ������ԣ�Ӧ�����γ��ܱ���ϵ����ˮ���м�ˮ��ʹˮ��Һ�����������Һ�棬����Һ��仯�жϣ�
��3��������Fe��Ӧ�����Ȼ���������˫��ˮ��������������Ϊ�����ӣ�
��4��ˮ�ܵ�Һ��ߣ�Ӧ�ý���ˮ�ܵĸ߶ȣ�
��5�����ݴ���Һ����ȡ���������ʵIJ���������
��6��Fe+2HCl=FeCl2+H2�����������������������ʵ������ٸ��ݷ���ʽ���Fe�����ʵ������������������Fe������������
��� �⣺��1����װ��ͼ��֪װ��AΪ��Һ©�����ʴ�Ϊ����Һ©����
��2������װ�õ������ԣ�Ӧ�����γ��ܱ���ϵ�����رջ���a�ͻ���b�����ɼ�K1��Ȼ����ˮ���м�ˮ��ʹˮ��Һ�����������Һ�棬һ��ʱ���ˮ����Һ�������������Һ�棬��Һ�����ȶ���˵�����������ã�
�ʴ�Ϊ�����رջ���a�ͻ���b��һ��ʱ���ˮ����Һ�������������Һ�棬��Һ�����ȶ���
��3��������Fe��Ӧ�����Ȼ�������ʵ��Ŀ�����Ʊ�FeCl3•6H2O���壬��Ҫ��˫��ˮ��������������Ϊ�����ӣ��䷴Ӧ�����ӷ���ʽΪH2O2+2Fe2++2H+=2Fe3++2H2O���ʴ�Ϊ��H2O2+2Fe2++2H+=2Fe3++2H2O��
��4����װ��ͼ��֪��ˮ�ܵ�Һ��ߣ�Ӧ�ý���ˮ�ܵĸ߶ȣ���Ӧ��ˮ�����ƣ��ʴ�Ϊ�����ƣ�
��5������Һ����ȡ���������ʵIJ���������Ũ������ȴ�ᾧ�����ˣ����õ�������Ϊ�������ձ����ƾ��ơ���������©��������Ҫ��Һ©����������
�ʴ�Ϊ��de��
��6��ʵ����������������ڹ��ռ���VmL���壬��n��H2��=$\frac{V}{{V}_{m}}$=$\frac{V}{22400}$mol����Fe+2HCl=FeCl2+H2����֪��n��Fe��=n��H2��=$\frac{V}{22400}$mol��
����m��Fe��=nM=$\frac{V}{22400}$mol��56g/mol=2.5V��10-3g��
��˷���м�������ʵ���������Ϊ��$\frac{2.5V��1{0}^{-3}g}{mg}$��100%=$\frac{2.5V��1{0}^{-3}}{m}$��100%��
�ʴ�Ϊ��$\frac{2.5V��1{0}^{-3}}{m}$��100%��
���� ���⿼�������ʵ��Ʊ������ʺ����IJⶨ�������ڿ������ʵ�������������ԭ��Ӧ�����ʺ������йؼ���ȣ��Ѷ��еȣ���Ҫѧ��������ʵ�Ļ���֪ʶ���������֪ʶ��������������

 ��У����ϵ�д�
��У����ϵ�д�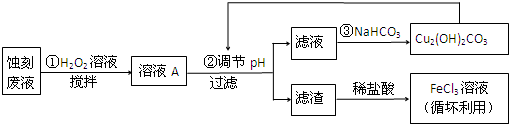
��ش�
��1��FeCl3-HCl��Һʴ��ͭ���ķ�Һ�к��еĽ�����������Fe3+��Fe2+��Cu2+��
��2��FeCl3ʴ��Һ�м��������Ŀ�ģ����������Ȼ���ˮ�⣬�ֿ����ʴ�����ʣ�
��3��������м���H2O2��Һ��Ŀ������Ϊ��Fe2+������Fe3+�������������ʱ��ȥ��
��4����֪��
�����������������pH
| Cu��OH��2 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 4.7 | 7.0 | 1.9 |
| ������ȫʱ | 6.7 | 9.0 | 3.2 |
��5��д�������������CO2��һ�����ӷ���ʽ4H++Cu2��OH��2CO3 =3H2O+2 Cu2++CO2������֪Cu2��OH��2CO3��������ˮ����
��6��д�����������Cu2��OH��2CO3�����ӷ���ʽ2Cu2++4HCO3-=Cu2��OH��2CO3��+H2O+3 CO2����
| A�� | ��ϵͳ������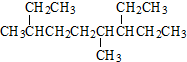 ������Ϊ��4��7-����-3-�һ����� ������Ϊ��4��7-����-3-�һ����� | |
| B�� | 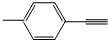 ����������ԭ�ӹ�ƽ�� ����������ԭ�ӹ�ƽ�� | |
| C�� | ����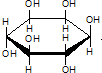 �������ǵ�Ԫ�������ͬ����ѧʽ��ΪC6H12O6������Cm��H2O��n����ˣ����߾������������ �������ǵ�Ԫ�������ͬ����ѧʽ��ΪC6H12O6������Cm��H2O��n����ˣ����߾������������ | |
| D�� | 1.0 mol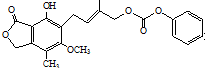 ������뺬5 mol NaOH��ˮ��Һ��ȫ��Ӧ ������뺬5 mol NaOH��ˮ��Һ��ȫ��Ӧ |
| A�� | Al2��SO4��3 | B�� | NaHCO3 | C�� | Fe��NO3��3 | D�� | NH4Cl |
| A�� | Al2��SO4��3=2Al+3+3SO4-2 | B�� | K2SO4=2K++S6++4O2- | ||
| C�� | CuCl2=Cu2++2Cl- | D�� | Na2CO3=Na++CO32- |
| A�� | BaSO4��ҽѧ���������ͣ�Ba2+�������� | |
| B�� | ��ѪҺ���������˽�������� | |
| C�� | ���Ӽ��������Ȼ�ѧ�����ö࣬�����������۵㡢�е��нϴ�Ӱ�죬�����ܽ����Ӱ�� | |
| D�� | ${\;}_{\;}^{14}$C��������������ļ�����${\;}_{\;}^{14}$C��${\;}_{\;}^{12}$C��Ϊͬ�������� |
| A�� | ������ˮ�м������NaOH���壬KW���� | |
| B�� | 0.1mol/LHF��Һ��PH=2�������Һ��c��OH-����c��HF�� | |
| C�� | ��Na2Sϡ��Һ�У�c��H+��=c��OH-��-2c��H2S��-c��HS-�� | |
| D�� | NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ |
 ��X��Y��Z��W��P��Q����ǰ�����ڵ�����Ԫ�أ�ԭ�������������۵�����֮��Ϊ26��ԭ�Ӱ뾶��Y��Z��W��P��Q��X���μ�С��Χ������Ԫ�أ��ش��������⣺
��X��Y��Z��W��P��Q����ǰ�����ڵ�����Ԫ�أ�ԭ�������������۵�����֮��Ϊ26��ԭ�Ӱ뾶��Y��Z��W��P��Q��X���μ�С��Χ������Ԫ�أ��ش��������⣺ ��YQ3����ԭ�ӵ��ӻ�����Ϊsp2��Z��Q��Ԫ�ص�һ�����ܵĴ�С��ϵ��Z��Q�����������������=������
��YQ3����ԭ�ӵ��ӻ�����Ϊsp2��Z��Q��Ԫ�ص�һ�����ܵĴ�С��ϵ��Z��Q�����������������=������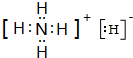 ������ˮ��Ӧ�Ļ�ѧ����ʽΪNH4H+H2O=NH3•H2O+H2����
������ˮ��Ӧ�Ļ�ѧ����ʽΪNH4H+H2O=NH3•H2O+H2����