题目内容
12. 有X、Y、Z、W、P、Q六种前两周期的主族元素,原子序数依次增大,价电子数之和为26,原子半径依Y、Z、W、P、Q、X依次减小.围绕上述元素,回答下列问题:
有X、Y、Z、W、P、Q六种前两周期的主族元素,原子序数依次增大,价电子数之和为26,原子半径依Y、Z、W、P、Q、X依次减小.围绕上述元素,回答下列问题:(1)Q的电子排布图为
 ,YQ3中心原子的杂化类型为sp2,Z与Q两元素第一电离能的大小关系:Z<Q(填“>”、“<”或“=”).
,YQ3中心原子的杂化类型为sp2,Z与Q两元素第一电离能的大小关系:Z<Q(填“>”、“<”或“=”).(2)X2P和ZP2固态时均为分子晶体,但熔点X2P比ZP2高得多,原因是H2O分子存在分子间氢键.
(3)固体A是离子晶体,结构类似于CsCl,组成中含W的质量分数为73.7%,它的所有原子的最外层都符合相应的稀有气体原子的最外层电子结构,该物质适当加热就分解成两种单质气体.该物质的电子式
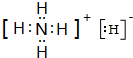 ,其与水反应的化学方程式为NH4H+H2O=NH3•H2O+H2↑.
,其与水反应的化学方程式为NH4H+H2O=NH3•H2O+H2↑.(4)Z单质有三类异形体,其中一种骨架型原子晶体的立方晶胞如图,计算晶体中Z原子的空间利用率为33.99%(√2√2=1.41,√3√3=1.73).
分析 有X、Y、Z、W、P、Q六种前两周期的主族元素,由原子序数X<Y,原子半径X<Y,可知X只能处于第一周期,其余元素处于第二周期,故X为H元素;Y、Z、W、P、Q原子序数依次增大,原子半径依Y、Z、W、P、Q依次减小,则最外层电子数依次增大,它们最外层电子数之和为26-1=25,最外层电子数只能分别为3、4、5、6、7,故Y为B、Z为C、W为N、P为O、Q为F;
(1)Q是F元素,根据核外电子排布画出其电子排布式;根据价层电子对互斥理论判断BF3中心原子B原子杂化方式;Z是C元素、Q是F元素,同一周期元素,元素第一电离能随着原子序数增大而呈增大趋势,但第IIA族、第VA族元素第一电离能大于其相邻元素;
(2)氢键导致物质的熔沸点升高;
(3)固体A是离子晶体,结构类似于CsCl,组成中含N的质量分数为73.7%,它的所有原子的最外层都符合相应的稀有气体原子的最外层电子结构,该物质适当加热就分解成两种单质气体,该物质为NH4H,与水反应生成氢气与一水合氨;
(4)该晶胞中C原子个数=4+8×1818+6×1212=8,设C原子半径为xcm,则C原子总体积=8×43π43πx3cm3,设正六面体棱长为ycm,其体线长=√3√3ycm,距离最近的C原子之间距离为2xcm,这两个碳原子之间的距离等于正六面体体长的1414,所以2xcm=√34y√34ycm,晶胞体积=y3cm3,空间利用率=原子总体积晶胞体积×100原子总体积晶胞体积×100.
解答 解:有X、Y、Z、W、P、Q六种前两周期的主族元素,由原子序数X<Y,原子半径X<Y,可知X只能处于第一周期,其余元素处于第二周期,故X为H元素;Y、Z、W、P、Q原子序数依次增大,原子半径依Y、Z、W、P、Q依次减小,则最外层电子数依次增大,它们最外层电子数之和为26-1=25,最外层电子数只能分别为3、4、5、6、7,故Y为B、Z为C、W为N、P为O、Q为F.
(1)Q为F元素,其电子排布图为 ,BF3中心原子B原子价层电子对数为3+1212(3-1×3)=3,故B原子采取sp2杂化;同一周期元素,元素第一电离能随着原子序数增大而呈增大趋势,但第IIA族、第VA族元素第一电离能大于其相邻元素,所以第一电离能C<F,
,BF3中心原子B原子价层电子对数为3+1212(3-1×3)=3,故B原子采取sp2杂化;同一周期元素,元素第一电离能随着原子序数增大而呈增大趋势,但第IIA族、第VA族元素第一电离能大于其相邻元素,所以第一电离能C<F,
故答案为: ;sp2杂化;<;
;sp2杂化;<;
(2)H2O和CO2固态时均为分子晶体,但H2O分子存在分子间氢键,熔点H2O比CO2高得多,
故答案为:H2O分子存在分子间氢键;
(3)固体A是离子晶体,结构类似于CsCl,组成中含N的质量分数为73.7%,它的所有原子的最外层都符合相应的稀有气体原子的最外层电子结构,该物质适当加热就分解成两种单质气体,该物质为NH4H,该物质的电子式为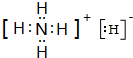 ,其与水反应的化学方程式为NH4H+H2O=NH3•H2O+H2↑,
,其与水反应的化学方程式为NH4H+H2O=NH3•H2O+H2↑,
故答案为: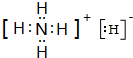 ;NH4H+H2O=NH3•H2O+H2↑;
;NH4H+H2O=NH3•H2O+H2↑;
(4)该晶胞中C原子个数=4+8×1818+6×1212=8,设C原子半径为xcm,则C原子总体积=8×43π43πx3cm3,设正六面体棱长为ycm,其体线长=√3√3ycm,距离最近的C原子之间距离为2xcm,这两个碳原子之间的距离等于正六面体体长的1414,所以2xcm=√34y√34ycm,所以xyxy=√38√38,晶胞体积=y3cm3,空间利用率=原子总体积晶胞体积×100原子总体积晶胞体积×100=8×43×π×x3y38×43×π×x3y3×100%=8×4343×π×(√38√38)3×100%=33.99%,故答案为:33.99%.
点评 本题考查物质结构和性质,涉及晶胞计算、电子式的书写、原子杂化方式判断、氢键等知识点,综合性较强,难点是晶胞计算,明确晶胞中距离最近的两个碳原子之间距离与晶胞体长关系是解本题关键,同时考查学生计算及空间想象能力,题目难度中等.

| A. | 向含有等物质的量的Ba(OH)2、KOH、的混合溶液中通入CO2;与CO2反应的物质依次是KOH、Ba(OH)2、BaCO3 | |
| B. | 向含有等物质的量的Fe2+、Ag+、Cu2+ 的混合溶液中加入Zn:与Zn反应的离子依次是Ag+、Cu2+、Fe2+ | |
| C. | 向含有等物质的量的AlO2?、OH-、CO32- 的混合溶液中滴加盐酸:与盐酸反应的物质依次是AlO2-、Al(OH)3、OH-、CO32- | |
| D. | 向含有等物质的量的AlCl3、HCl的混合溶液中滴加NaOH溶液,与NaOH反应的物质依次是AlCl3、HCl、Al(OH)3 |
| A. | 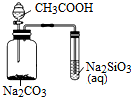 不能用来检验CH3COOH、H2CO3、H2SiO3酸性的强弱 | |
| B. | 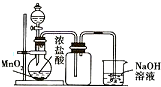 可用来制取并收集氯气 | |
| C. | 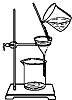 可用来分离氢氧化铁胶体中的胶体粒子 | |
| D. | 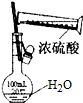 可用来配制一定物质的量浓度的稀硫酸 |
| A. | 每消耗1molCH4可以向外电路转移4mol电子 | |
| B. | 负极上CH4失去电子,电极反应式为CH4+10OH--8e-═CO32-+7H2O | |
| C. | 负极上是O2获得电子,电极反应式为:O2+2H2O+4e-═4OH- | |
| D. | 电池放电后,溶液pH不断升高 |
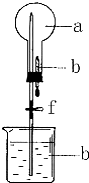 如图的装置中,干燥烧瓶中盛有某种气体,烧杯和滴管内盛放某种溶液.挤压滴管的胶头,下列与试验事实不相符的是( )
如图的装置中,干燥烧瓶中盛有某种气体,烧杯和滴管内盛放某种溶液.挤压滴管的胶头,下列与试验事实不相符的是( )| A. | CO2(NaHCO3溶液)无色喷泉 | B. | NH3(H2O含酚酞)红色喷泉 | ||
| C. | Cl2(NaOH溶液)无色喷泉 | D. | HCl(AgNO3溶液)白色喷泉 |
| A. | +30.67 | B. | -345.3 | C. | -30.67 | D. | +345.3 |
| A. | 常温常压下,3.0g含甲醛的冰醋酸中含有的原子总数为0.4 NA | |
| B. | 标准状况下,2.24L苯中含有的C-H键的数目为0.6NA | |
| C. | 1 L 0.01 mol•L-1 KAl(SO4)2溶液中,含有的阳离子数目为0.02NA | |
| D. | 反应KIO3+6HI=3I2+KI+3H2O,每生成1.5mol I2转移电子数为3NA |
| A. | 电解过程产生的气体体积(在标准状况下)为5.6 L | |
| B. | 电解过程只发生了2CuSO4+2H2O电解_电解–––––2Cu+O2↑+2H2SO4 | |
| C. | 电解过程转移的电子数为3.612×1023个 | |
| D. | 加入的碱式碳酸铜的反应是:Cu2(OH)2CO3+2H2SO4═2CuSO4+CO2↑+3H2O |
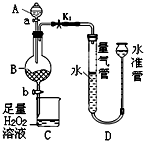 工业生产中会产生大量的废铁屑,可将其“变废为宝”制成化工原料氯化铁.实验室中利用如图所示装置探究由废铁屑制备FeCl3•6H2O晶体的原理并测定铁屑中铁单质的质量分数(杂质不溶于水且不与酸反应).
工业生产中会产生大量的废铁屑,可将其“变废为宝”制成化工原料氯化铁.实验室中利用如图所示装置探究由废铁屑制备FeCl3•6H2O晶体的原理并测定铁屑中铁单质的质量分数(杂质不溶于水且不与酸反应).