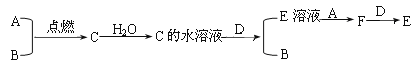��Ŀ����
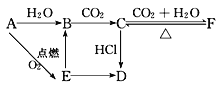
����Ŀ���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�

(1)д���������������ƣ���________����________����________��
(2)������װ�â�������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ�����______��������������������е�ʵ�����������Ϊ________�������ܵĽ�ˮ����________(����f������g��)
(3)ʵ������NaOH��������250mL 1.25mol/L��NaOH��Һ����ղ���ش��������⣺
������ʱ������ȷ�IJ���˳����(��ĸ��ʾ��ÿ����ĸֻ����һ��)_________________��
A.��30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B.����ƽȷ��ȡ�����NaOH����������������ˮ(Լ30mL)���ò���������������ʹ�����ܽ�
C.������ȴ��NaOH��Һ�ز�����ע��250mL������ƿ��
D.������ƿ�ǽ����ߵ�ҡ��
E.���ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F.����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
���������Ƶ���ҺŨ��ƫ�͵���_________________��
A.����NaOHʱ���������������
B.������ƿ��ת����Һʱ(ʵ�鲽��C)������Һ����������ƿ����
C.������ˮʱ���������˿̶���
D.����ʱ���ӿ̶���
E.����ǰ������ƿ������������ˮ
��װ�â���ijͬѧת����Һ��ʾ��ͼ��ָ��ͼ�еĴ����ǣ�___________��____________��
(4)�����к�Ca2+��Mg2+��SO42-�����ʣ���Ҫ�ᴿ������ۺ����á������ᴿ�IJ����У��ټ��������Na2CO3��Һ���ڼ��������BaCl2��Һ���ۼ��������NaOH��Һ���ܼ���������������ܽ⣻���ˣ�����������ȷ�IJ���˳����__________(��д�����ĸ)��
a.�ݢ٢ڢۢޢܢ� b.�ݢڢ٢ۢܢޢ� c.�ݢۢڢ٢ޢܢ�
���𰸡�������ƿ ��ƿ ����ƿ �¶ȼ� ����(�����) g BCAFED ABC δ�ò��������� δ����250 mL����ƿ c
��������
(1)���������Ľṹ�ص��жϣ�
(2)�������Ȼ�̼�;ƾ��Ļ��������þƾ��Ƽ��ȣ�ʵ����������̣�����Ҫʹ���¶ȼƣ��¶ȼ�Ӧλ��������ƿ֧�ܿڣ��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
(3)�ٸ�������һ�����ʵ���Ũ�ȵ���Һ�ķ����Ͳ����жϲ���˳��
�ڸ���c=![]() ����ʵ����
����ʵ����
�۸�������һ�����ʵ���Ũ�ȵ���Һ�ķ����жϣ�
(4)��ʵ�����̿�֪����ˮɹ�η�������Σ������к�Ca2+��Mg2+��SO42-�����ʣ�ѡNaOH��ȥþ���ӣ�ѡ�Ȼ�����ȥ��������ӣ�ѡ̼���Ƴ�ȥ�����Ӽ������ı����ӣ���̼����һ�����Ȼ���֮��
(1)���������ṹ��֪����Ϊ������ƿ����Ϊ��ƿ����Ϊ����ƿ��
(2)�������Ȼ�̼�;ƾ��Ļ����������ķ������룬�����þƾ��Ƽ��ȣ����¶ȼƲ����������¶ȣ��¶ȼ�ˮ����Ӧλ��������ƿ֧�ܿڣ�Ϊ�˳��������Ҫ��������ԭ�����������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ��������ܵĽ�ˮ��Ϊg��
(3)������һ�����ʵ���Ũ�ȵ���Һ�IJ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ�����ʵ������NaOH��������250mL 1.25mol/L��NaOH��Һ�IJ�����BCAFED��
������250mL 1.25mol/L��NaOH��Һ����ҪNaOH��������m(NaOH)=1.25mol/L��0.25L��40g/mol=12.5g��
A.����NaOHʱ��������������̣���ʵ�ʳ�������Ϊ12g-0.5g=11.5g������ƫ�٣������Ƶ���ҺŨ��ƫ�ͣ�A�������⣻
B.������ƿ��ת����Һʱ(ʵ�鲽��C)������Һ����������ƿ���棬�������ʼ��٣�ʹ���Ƶ���ҺŨ��ƫ�ͣ�B�������⣻
C.������ˮʱ���������˿̶��ߣ�����Һ�����ƫ�������������ʵ���û�б仯�������Ƶ���ҺŨ��ƫ�ͣ�C�������⣻
D.������ʱ���ӿ̶��ߣ���VƫС���������Ƶ���ҺŨ��ƫ�ߣ�D���������⣻
E.������ǰ������ƿ������������ˮ�����ڲ�Ӱ����Һ���������˶����Ƶ���ҺŨ����Ӱ�죬D���������⣻
�ʺ���ѡ����ABC��
������һ�����ʵ���Ũ�ȵ���Һת����Һʱ�����ò�������������ֹҺ���⽦��δʹ�ò���������������250mL 1.25mol/L��NaOH��Һ����Ҫʹ��250mL������ƿ������ʹ��500ml����ƿ��
(4)��ʵ�����̿�֪����ˮɹ�η�������Σ������к�Ca2+��Mg2+��SO42-�����ʣ�ѡNaOH��ȥþ���ӣ�ѡ�Ȼ�����ȥ��������ӣ�ѡ̼���Ƴ�ȥ�����Ӽ������ı����ӣ�̼����һ�����Ȼ���֮���˺��ټ��������ᣬ��������õ�NaCl������ȷ�IJ���˳��Ϊ�ݢڢۢ٢ޢܢ�ݢۢڢ٢ޢܢߣ�����ȷ˳��Ϊc��

 53���ò�ϵ�д�
53���ò�ϵ�д�