��Ŀ����
ʵ���ҿ�����MgCl2?6H2OΪԭ���Ʊ�̼��þ���룬��Ҫ����������
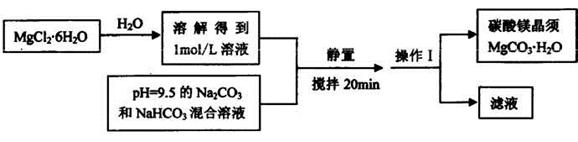
�Իش�
(1)����1��������_______��
(2)������Ӧ����Ҫ���ƺ��¶ȣ���ΪMgCO3����ˮ�г�ʱ�������п���ȫ����Mg(OH)2����ԭ����_____________________��
(3)��������ף��ڼ������ʱҲ�������ɼ�ʽ̼��þMg5(OH)2(CO3)4��4H2O��д���÷�Ӧ�Ļ�ѧ����ʽ______________��
(4)ij������Ϊ��ȷ��MgCO3��ˮ�г�ʱ�������к����õĹ���ɷ֣����������о�����ע��Mg(OH)2, Mg5(OH)2(CO3)4.4H2O�����ʱ������ֽ⣩
�ٶ����о�����������±������ݡ�
����ȡһ�������Ĺ��壬���������أ��ܷ�ͨ�����������ļ�������ȷ������ijɷ֣�_______ (��ܡ���)��������______________

�Իش�
(1)����1��������_______��
(2)������Ӧ����Ҫ���ƺ��¶ȣ���ΪMgCO3����ˮ�г�ʱ�������п���ȫ����Mg(OH)2����ԭ����_____________________��
(3)��������ף��ڼ������ʱҲ�������ɼ�ʽ̼��þMg5(OH)2(CO3)4��4H2O��д���÷�Ӧ�Ļ�ѧ����ʽ______________��
(4)ij������Ϊ��ȷ��MgCO3��ˮ�г�ʱ�������к����õĹ���ɷ֣����������о�����ע��Mg(OH)2, Mg5(OH)2(CO3)4.4H2O�����ʱ������ֽ⣩
�ٶ����о�����������±������ݡ�
| ʵ�鲽�� | Ԥ�ڵ�ʵ������ͽ��� |
| ȡһ�������Ĺ�����Ʒ | �� ����������ΪMg(OH)2 |
����ȡһ�������Ĺ��壬���������أ��ܷ�ͨ�����������ļ�������ȷ������ijɷ֣�_______ (��ܡ���)��������______________
��14�֣�
��1�����ˣ�2�֣�
��2��MgCO3�ڼ�����й������ܷ���ˮ�ⷴӦ������ˮ�����ɵ�Mg(OH)2�ܽ�ȸ�С��������CO2�������ɣ�ʹˮ��������ȫ�� MgCO3ת��ΪMg(OH)2�� ��3�֣�
��3�� 5 MgCO3 + 5H2O=Mg5(OH)2(CO3)4��4H2O + CO2�� ��2�֣�
��4���� ��4�֣�
�����������𰸾����֣�
�� �ܣ�1�֣� ��������������þ���ʽ̼��þ����������ʱ�����������ļ�������ȷ���ģ��Ҳ���ͬ����2�֣�
��1�����ˣ�2�֣�
��2��MgCO3�ڼ�����й������ܷ���ˮ�ⷴӦ������ˮ�����ɵ�Mg(OH)2�ܽ�ȸ�С��������CO2�������ɣ�ʹˮ��������ȫ�� MgCO3ת��ΪMg(OH)2�� ��3�֣�
��3�� 5 MgCO3 + 5H2O=Mg5(OH)2(CO3)4��4H2O + CO2�� ��2�֣�
��4���� ��4�֣�
| ʵ�鲽�� | Ԥ�ڵ�ʵ������ͽ��� |
| �����Թ��У��μ�������ϡ���� ���������Թ��г�ּ��ȣ�������������ͨ�뵽ʢ����������ʯ��ˮ���ձ��� | ���������� �������ʯ��ˮ������� �� |
�����������𰸾����֣�
�� �ܣ�1�֣� ��������������þ���ʽ̼��þ����������ʱ�����������ļ�������ȷ���ģ��Ҳ���ͬ����2�֣�
����������ڽ��̽�����ʱ��Ҫ�ܹ��������л�ȡ���֪ʶ��ϵ�α�֪ʶ���н��⡣���⽫Mg2+��CO32-�������MgCO3��������̼���ˮ��ʼ��ԺͿα���ˮ�������ɵ����֪ʶ����֪��ת��ΪMg(OH)2�Լ���Ŀ���ᵽ��Mg5(OH)2(CO3)4��4H2O��������������ʲô���������̽����̽���Ľ�������������̼����ļ��飬�Ӷ����⡣

��ϰ��ϵ�д�
 ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ
 CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������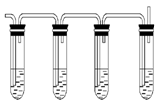
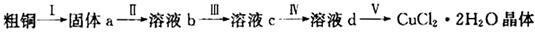
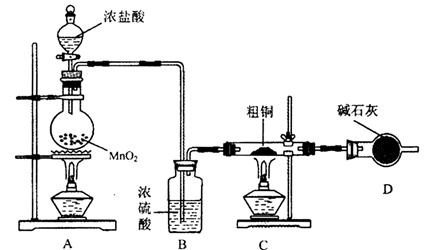
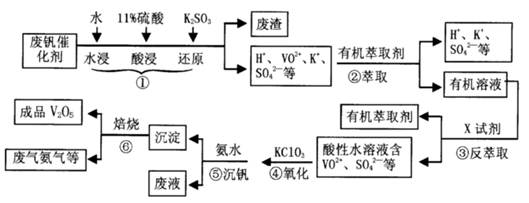
 2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��
2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��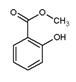 �����ж���ζ�������������ͣ���Ҫ��������ɱ�����ɱ���������ϡ�Ϳ�ϵȡ���ˮ���ᣨ
�����ж���ζ�������������ͣ���Ҫ��������ɱ�����ɱ���������ϡ�Ϳ�ϵȡ���ˮ���ᣨ ����״��������õĶ����������������δ��Ӧ��ȫ��ˮ���ᣬ��Ҫ�����ᴿ�����˵�ϴ�Ӽ�Ϊ�� ��
����״��������õĶ����������������δ��Ӧ��ȫ��ˮ���ᣬ��Ҫ�����ᴿ�����˵�ϴ�Ӽ�Ϊ�� ��