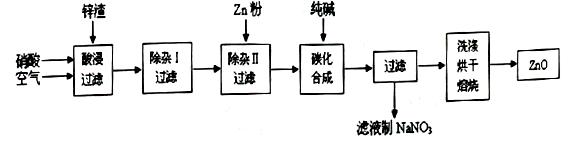
��Ŀ����
�ӷϷ���������Ҫ�ɷ�V2O5��VOSO4��K2SO4��SiO2�ȣ��л���V2O5��һ��������������ʾ��ͼ���£���ش��������⣺
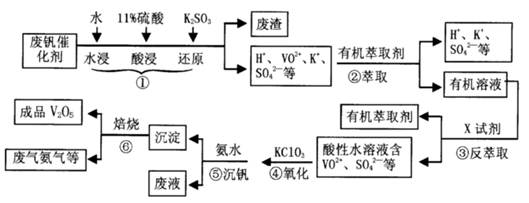
��1��������з�������Ҫ�ɷ��� ������X�Լ�Ϊ ��
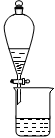
��2��ʵ�����н�����ȡ��Һ����ʱ��ע����ȡ�������������Һ©������Ȧ�Ͼ��ã���Һ��ֲ�������IJ����� ��
��3���ڡ��۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO2+��HA��ʾ�л���ȡ������
R2(SO4)n (ˮ��)+ 2nHA���л��㣩 2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��
2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��
��4������ɢ��еķ�Ӧ���ӷ���ʽ��
��ClO3- + ��VO2+ +��H+ =��VO3+ + �� +��
��5��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
����ϱ�����ʵ�������У����м��백ˮ��������Һ�����pHֵΪ ��
��6���ù��������У�����ѭ�����õ������� ��

��1��������з�������Ҫ�ɷ��� ������X�Լ�Ϊ ��
��2��ʵ�����н�����ȡ��Һ����ʱ��ע����ȡ�������������Һ©������Ȧ�Ͼ��ã���Һ��ֲ�������IJ����� ��
��3���ڡ��۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO2+��HA��ʾ�л���ȡ������
R2(SO4)n (ˮ��)+ 2nHA���л��㣩
 2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��
2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ����4������ɢ��еķ�Ӧ���ӷ���ʽ��
��ClO3- + ��VO2+ +��H+ =��VO3+ + �� +��
��5��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
����ϱ�����ʵ�������У����м��백ˮ��������Һ�����pHֵΪ ��
��6���ù��������У�����ѭ�����õ������� ��
��SiO2��2�֣� H2SO4 ��2�֣�
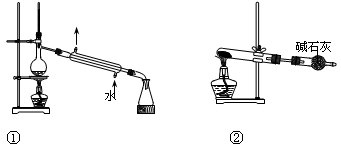
���ڷ�Һ©���·���һ�ྻ�ձ���ʹ©���¶˹ܿڽ����ձ��ڱڣ�����Һ©���Ͽ����ӣ���Һ©���Ͽ����Ӱ��۶�©���ھ���С�ף������������²�Һ������ձ�������Һ��ӽ������Գ�����������ʱ�رջ������ϲ�Һ��ӷ�Һ©���Ͽڵ�����һ�ձ��С���2�֣�Ҫ�㣺��Һ©���Ͽ����ӣ���Һ©���Ͽ����Ӱ��۶�©���ھ���С�ף���1�֣����ȴ����ų��²�Һ�壬���ϲ�Һ��ӷ�Һ©���Ͽڵ�����һ�ձ��У�1�֣���
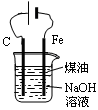
�Ǽ�����к�����ʹƽ�����ơ����������ȡ��2�֣� д������������NaOH��KOH����ˮ���к�����ʹƽ�����ơ�����2�֣�д�����������ȡ������2�֣�д������Mg��Fe������ǿ���Ρ�������2�֣�д��������ȡ���ļ�����������1�֣�д��������ȡ��Ũ�ȡ����÷֣�д���Ƴ�H2SO4 ����1�֡�
��1ClO3- + 6VO2+ + 6H+ = 6VO3+ + 1Cl- + 3H2O ��2�֣�����Cl-��H2O��1�֡���ƽ��ȷ��1�֡�
��д��1������1�֡�
��1.7��1.8��2�֣� д1.7��1.8��1.75��1.7��1.8֮�����2�֡�
д��1.6����1�֡�
�ʰ��� �л���ȡ����2�֣���1�֡�ÿ��дһ����1�֡�
���ڷ�Һ©���·���һ�ྻ�ձ���ʹ©���¶˹ܿڽ����ձ��ڱڣ�����Һ©���Ͽ����ӣ���Һ©���Ͽ����Ӱ��۶�©���ھ���С�ף������������²�Һ������ձ�������Һ��ӽ������Գ�����������ʱ�رջ������ϲ�Һ��ӷ�Һ©���Ͽڵ�����һ�ձ��С���2�֣�Ҫ�㣺��Һ©���Ͽ����ӣ���Һ©���Ͽ����Ӱ��۶�©���ھ���С�ף���1�֣����ȴ����ų��²�Һ�壬���ϲ�Һ��ӷ�Һ©���Ͽڵ�����һ�ձ��У�1�֣���
�Ǽ�����к�����ʹƽ�����ơ����������ȡ��2�֣� д������������NaOH��KOH����ˮ���к�����ʹƽ�����ơ�����2�֣�д�����������ȡ������2�֣�д������Mg��Fe������ǿ���Ρ�������2�֣�д��������ȡ���ļ�����������1�֣�д��������ȡ��Ũ�ȡ����÷֣�д���Ƴ�H2SO4 ����1�֡�
��1ClO3- + 6VO2+ + 6H+ = 6VO3+ + 1Cl- + 3H2O ��2�֣�����Cl-��H2O��1�֡���ƽ��ȷ��1�֡�
��д��1������1�֡�
��1.7��1.8��2�֣� д1.7��1.8��1.75��1.7��1.8֮�����2�֡�
д��1.6����1�֡�
�ʰ��� �л���ȡ����2�֣���1�֡�ÿ��дһ����1�֡�
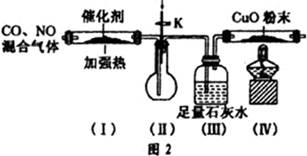
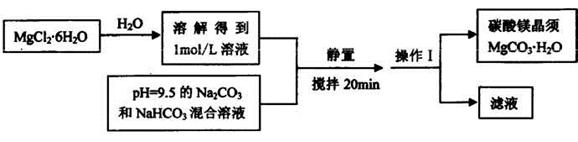
�������������������Ĺؼ��Ǹ��������ʲô���ʣ�����ʲô��Ӧ����η�������⣬����������ת��Ϊ���ʵ����̡���SiO2������ˮ���ᣬͨ�����˳�ȥ���ڷ����У�V2O5����ԭΪVO2+������������һ���������Һ�У��ڴӷ����п��Կ�����VO2+�������л��ܼ���ͨ����ȡ���룻��ͨ������ȡ���õ�����ˮ��Һ��VO2+��SO42-����XΪH2SO4��Ȼ��ͨ��������V��+4��+5�����백ˮ���ʼ���ת��Ϊ������ͨ�����գ��õ�V2O5�������������̲������ӡ�

��ϰ��ϵ�д�
 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
�����Ŀ
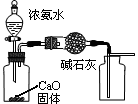
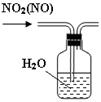
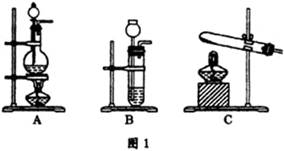
 CO+H2O��ʵ��������ͼl��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��Ϊ
CO+H2O��ʵ��������ͼl��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��Ϊ