��Ŀ����
����Ŀ����1����H2��g��+![]() O2��g��= H2O��l�� ��H= ��285.8 kJ/mol
O2��g��= H2O��l�� ��H= ��285.8 kJ/mol
��H2��g��+![]() O2��g��= H2O��g�� ��H= ��241.8kJ/mol
O2��g��= H2O��g�� ��H= ��241.8kJ/mol
��C(s)+![]() O2 (g) = CO (g) ��H= ��110.5kJ/mol
O2 (g) = CO (g) ��H= ��110.5kJ/mol
��C(s)+ O2 (g) = CO2 (g) ��H= ��393.5kJ/mol
�ش��������⣺
������Ӧ�����ڷ��ȵ���_________��H2��ȼ����Ϊ________��C��ȼ����Ϊ_____��
��2�����͵���Ҫ�ɷ������飨C8H18����1 mol C8H18��l����O2��g����ȼ�գ�����CO2��g����H2O��l�����ų�5518 kJ��������д���˷�Ӧ���Ȼ�ѧ����ʽ___________
��3����������������Ӧ����1molˮ��������241.8kJ����1gˮ����ת����Һ̬ˮʱ����2.444kJ����д��������ȼ���ȵ��Ȼ�ѧ����ʽ________
���𰸡��٢ڢۢ� 285.8 kJ/mol 393.5 kJ/mol C8H18��l��+![]() O2��g��=8CO2��g��+9H2O��l�� ��H=-5518kJ/mol H2(g)+
O2��g��=8CO2��g��+9H2O��l�� ��H=-5518kJ/mol H2(g)+![]() O2(g)=H2O (l) ��H=-285.792kJ/mol
O2(g)=H2O (l) ��H=-285.792kJ/mol
��������
��1��������Ӧ���ǡ�H��0�ķ�Ӧ�����Ƿ��ȷ�Ӧ���ʴ�Ϊ���٢ڢۢܣ�
������ȼ������ָ1mol����ȼ������Һ̬ˮʱ�ų���������C��ȼ������ָ1molCȼ�����ɶ�����̼ʱ�ų�����������Ϸ�Ӧ�٢ܿ�֪��������ȼ����Ϊ285.8kJ/mol��C��ȼ����Ϊ��393.5kJ/mol���ʴ�Ϊ��285.8kJ/mol��393.5kJ/mol��
��2��1molC8H18��l������O2��g����ȼ�գ�����CO2��g����H2O��l�����ų�5518kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪC8H18��l��+![]() O2��g��=8CO2��g��+9H2O��l����H=-5518kJ/mol��
O2��g��=8CO2��g��+9H2O��l����H=-5518kJ/mol��
��3��������������Ӧ����1molˮ��������241.8kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��H2��g��+![]() O2��g��=H2O��g����H=-241.8kJ/mol��
O2��g��=H2O��g����H=-241.8kJ/mol��
1gˮ����ת����Һ̬ˮ����2.444kJ����18gˮ����ת����Һ̬ˮ�ų�����2.444kJ��18=43.992kJ���ʷ�ӦH2��g��+![]() O2��g��=H2O��l���ķ�Ӧ�ȡ�H=-��241.8kJ/mol+43.992kJ/mol��=-285.792kJ/mol��������ȼ���ȷ���ʽΪ��H2��g��+
O2��g��=H2O��l���ķ�Ӧ�ȡ�H=-��241.8kJ/mol+43.992kJ/mol��=-285.792kJ/mol��������ȼ���ȷ���ʽΪ��H2��g��+![]() O2��g��=H2O��l����H=-285.792kJ/mol��
O2��g��=H2O��l����H=-285.792kJ/mol��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����֪��ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ���������������ԭ������ش��������⣺
��1�����淴ӦFeO(s)��CO(g)![]() Fe(s)��CO2(g)��������ҵ��һ����Ҫ��Ӧ�����¶���ƽ�ⳣ��K�Ĺ�ϵ���±���
Fe(s)��CO2(g)��������ҵ��һ����Ҫ��Ӧ�����¶���ƽ�ⳣ��K�Ĺ�ϵ���±���
T/K | 938 | 1 100 |
K | 0.68 | 0.40 |
���÷�Ӧ������̶����ܱ������н��У���һ�������´ﵽƽ��״̬���������¶ȣ���������ƽ����Է�������__�����뺤�������������ܶ�__(��������������С������������)��
��2��830�棬��ӦCO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)��ƽ�ⳣ��K=1����2L���ݷ�Ӧ���з���������Ӧ���ֱ����3molCO2��2molH2��1molCH3OH��4molH2O(g)����Ӧ��__������С�������������桱���ƶ�����
CH3OH(g)��H2O(g)��ƽ�ⳣ��K=1����2L���ݷ�Ӧ���з���������Ӧ���ֱ����3molCO2��2molH2��1molCH3OH��4molH2O(g)����Ӧ��__������С�������������桱���ƶ�����
��3��25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
��ѧʽ | CH3COOH | NH3��H2O | H2CO3 | H2SO3 |
����ƽ�ⳣ�� | 1.7��10-5 | 1.7��10-5 | K1��4.3��10-7 K2��5.6��10-11 | K1��1.3��10-2 K2��6.3��10-8 |
��д��CH3COOH�ĵ��뷽��ʽ____�������ƽ�ⳣ������ʽK a=___��
�ڸ��ݱ��������жϣ�Ũ�Ⱦ�Ϊ0.01mol��L-1�������������ʵ���Һ�У�������ǿ����___(����)�������и���Һ�ֱ�ϡ��100����pH�仯��С����___(����)��
A��CH3COOH B��H2CO3 C��H2SO3
����Ŀ��I.��1���������з�Ӧ��2SO2 + O2 ![]() 2SO3 �� ���2min��SO2��Ũ����8 mol/L�½�Ϊ2 mol/L����ô����SO2Ũ�ȱ仯����ʾ�Ļ�ѧ��Ӧ����Ϊ____����O2Ũ�ȱ仯����ʾ�ķ�Ӧ����Ϊ_____________�������ʼʱSO2Ũ��Ϊ4mol/L��2min��Ӧ��ƽ�⣬�����ʱ����v(O2)Ϊ0.5mol/(L��min)����ô2minʱSO2��Ũ��Ϊ_____________��
2SO3 �� ���2min��SO2��Ũ����8 mol/L�½�Ϊ2 mol/L����ô����SO2Ũ�ȱ仯����ʾ�Ļ�ѧ��Ӧ����Ϊ____����O2Ũ�ȱ仯����ʾ�ķ�Ӧ����Ϊ_____________�������ʼʱSO2Ũ��Ϊ4mol/L��2min��Ӧ��ƽ�⣬�����ʱ����v(O2)Ϊ0.5mol/(L��min)����ô2minʱSO2��Ũ��Ϊ_____________��
��2����ͼ��ʾ���ܱ������з�Ӧ��2SO2+O2![]() 2SO3 ��H<0 �ﵽƽ��ʱ�����������ı������Ӧ�ٶȺͻ�ѧƽ��ı仯�����a b�����иı������������____________��b c�����иı������������____________�� ������ѹǿʱ����Ӧ�ٶȱ仯�������c-d��__________.
2SO3 ��H<0 �ﵽƽ��ʱ�����������ı������Ӧ�ٶȺͻ�ѧƽ��ı仯�����a b�����иı������������____________��b c�����иı������������____________�� ������ѹǿʱ����Ӧ�ٶȱ仯�������c-d��__________.
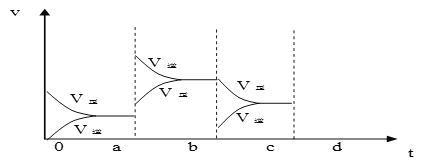
��3���������е���ƽ�⣺NH3��H2O![]() NH4+ + OH -����ʹc(NH4+)����Ĵ�ʩ�ǣ�_________
NH4+ + OH -����ʹc(NH4+)����Ĵ�ʩ�ǣ�_________
�������¶� �ڼӰ��� ��ˮ �� NH4Cl��Һ ��NaOH��Һ ������������
II.��һ��������ܱ������У��������»�ѧ��Ӧ��CO2��g����H2��g��![]() CO��g����H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
CO��g����H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK��____________��
��2���÷�ӦΪ____________��Ӧ��ѡ�����ȡ����ȣ���
��3��ij�¶��£�ƽ��Ũ�ȷ�����ʽ��3c��CO2����c��H2����5c��CO����c��H2O�������жϴ�ʱ���¶�Ϊ____________�棻
��4��830��ʱ����������м���1LCO2��1LH2��ƽ��ʱCO2�����������____________��