��Ŀ����
����Ŀ�����ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa;X��Y������缫��(X��Y���Ƕ��Ե缫)��A��B�dz��ڣ�ͨ��������ֱ����Դ��������ش��������⣺

��1����a��CuCl2��Һ����
��X�ĵ缫������___________���缫��Ӧʽ��_________________________��
����B���ڷ�һ��ʪ���KI-������ֽ���Ϊ__________ɫ��
��2����a����NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
��������X���ϵĵ缫��Ӧʽ��______________����X�������۲쵽��������_____________��
���õ����ܷ�Ӧʽ��___________________________________��
��3����a��400mL2mol/LCuSO4��Һ��һ��ʱ����������1.28g(�ü�������ų�)��������Һ������䣬����Һ��pHΪ__________���������ռ����������__________mL��
���𰸡� ���� Cu2++2e-=Cu �� 2H2O+2e-=H2��+2OH- ��ɫ��Һ��� 2NaCl+2H2O![]() 2NaOH+H2��+Cl2�� 1 224
2NaOH+H2��+Cl2�� 1 224
����������1����a��CuCl2��Һ������X���ӵ�Դ�ĸ������缫������������������ͭ���ӵõ�������ͭ���缫��Ӧʽ��Cu2++2e-=Cu����������������ʧ���Ӳ�����������B���ڷ�һ��ʪ���KI-������ֽ���Ϊ��ɫ����2����a����NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ���ٵ�����X��Ϊ������������ˮ��������������ӵõ��Ӳ����������缫��Ӧʽ��2H2O+2e-=H2��+2OH-����X���������������ģ�����������Ũ�����۲쵽����������ɫ��Һ��죻�õ���Ϊ����Ȼ�����Һ���ܷ�Ӧʽ��2NaCl+2H2O![]() 2NaOH+H2��+Cl2������3����a��400mL 2mol/LCuSO4��Һ������������Cu�����������������ӷŵ�������������ط�ӦʽΪ2Cu 2++2H2O
2NaOH+H2��+Cl2������3����a��400mL 2mol/LCuSO4��Һ������������Cu�����������������ӷŵ�������������ط�ӦʽΪ2Cu 2++2H2O![]() 4H++2Cu+O2��������Cu��H+֮��Ĺ�ϵʽ����c��H+��=
4H++2Cu+O2��������Cu��H+֮��Ĺ�ϵʽ����c��H+��= =0.1mol/L������Һ��pH=1������ת�Ƶ�����ȵ��������=
=0.1mol/L������Һ��pH=1������ת�Ƶ�����ȵ��������= ��1��22.4L/mol=224mL��
��1��22.4L/mol=224mL��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������л���A��B���Ի��ܣ��й��������£�
����ܶ�(20��) | �۵� | �е� | �ܽ��� | |
A | 0.7893 | -117.3�� | 78.5�� | ��ˮ������Ȼ��� |
B | 0.7137 | -116.6�� | 34.5�� | ������ˮ |
��1����Ҫ��ȥA��B�Ļ������������B���ɲ���_______(�����)�������ɵõ�A��
a���ؽᾧ b������ c����ȡ d����ˮ�������Һ
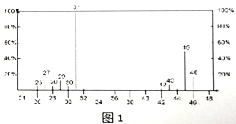


��2�����л���A�����������г��ȼ�գ�ʵ���ã�����5.4gH2O��8.8gCO2����������6.72L(��״����)��������ʵ����ʽΪ_________����Ҫȷ�������ʽ���Ƿ��������������_______(����������������)����֪�л���A�����ס��˴Ź���������ͼ1��ʾ����A�Ľṹ��ʽΪ________��
��3��������ͼ2��ʾB����Է�������Ϊ74,���������ͼ3��ʾ����B�Ľṹ��ʽΪ_________��������ŵ�����Ϊ_________��
��4��ȷ��ȡһ��������A��B�Ļ��������������г��ȼ�գ�����������ͨ����������ˮ�Ȼ��ƺͼ�ʯ�ң����������ֱ�����19.8g��35.2g������������A��B�����ʵ���֮��_________��