��Ŀ����
17������������������ȷ���ǣ�������| A�� | 2NO2��g��������ɫ��?N2O4��g������ɫ����H��0����ƽ���ƽ����ϵ��ȡ��С�ݻ�������ѹǿ�Ĵ�ʩ����Ϊƽ��������Ӧ�����ƶ�������ϵ��ɫ��dz | |
| B�� | ����N2��g��+3H2��g��?2NH3��g����ƽ�����ѹǿ���䣬����Ar��ƽ������ | |
| C�� | FeCl3+3KSCN?Fe��SCN��3����ɫ��+3KCl����ƽ�������KCl���壬��Ϊƽ�����淴Ӧ�����ƶ�������ϵ��ɫ��dz | |
| D�� | H2��g��+I2��g��?2HI��g����H��0����ƽ���ƽ����ϵ��ȡ�����ݻ�����Сѹǿ�Ĵ�ʩ����Ϊƽ�ⲻ�ƶ�������ϵ��ɫ���� |
���� A����С�ݻ�������ѹǿƽ�������������С�ķ����ƶ�����ϵ��ɫ�����������Ũ�ȳ����ȣ��Դ˽��
B���ϳɰ�ʱ����ѹǿ���䣬����Ar������������൱�ڷ�Ӧ��ϵ��ѹǿ��С��ƽ�������������ķ����ƶ���
C�������Ӳ����뷴Ӧ��
D�������ݻ�����Сѹǿ��ƽ�ⲻ�����ƶ�����������������ɫ��dz��
��� �⣺A����С�ݻ�������ѹǿƽ�������������С�ķ����ƶ�������Ӧ�����ƶ���������������Ũ����Ȼ����������ϵ��ɫ�����A����
B���ϳɰ�ʱ����ѹǿ���䣬����Ar������������൱�ڷ�Ӧ��ϵ��ѹǿ��С��ƽ�������������ķ����ƶ�������ƽ�����ƣ���B��ȷ��
C�������Ӳ����뷴Ӧ��ƽ�ⲻ�ƶ�����C����
D�������ݻ�����Сѹǿ��ƽ�ⲻ�����ƶ�����������������ɫ��dz����D����
��ѡB��
���� ���⿼����ƽ���ƶ�ԭ�����ѶȲ���ע��ʹ����������ԭ����Ӧ�ã��״���ΪB��

��ϰ��ϵ�д�
�����Ŀ
5�����и������ﲻ���÷�Һ©��������ǣ�������
| A�� | ��������ˮ | B�� | ���ͼױ� | C�� | �ұ���ˮ | D�� | �屽��NaOH��Һ |
7�����ʽṹ�������ʣ������о����ʵ��۽ṹ���������������ʱ仯�ı��ʣ���ش��������⣺
��1��C��Si��N�ĵ縺���ɴ�С��˳����N��C��Si��Na��Mg��Al�ĵ�һ�������ɴ�С��˳����Mg��Al��Na��
��2��A��B��Ϊ�����ڽ���Ԫ�أ����ݱ��е����ݣ�д��Bԭ�ӵĵ����Ų�ʽ��1s22s22p63s2��
��3�����ɽ���������ˮ�����γɵ�������Ƿ�����ɫ������d��������Ų��йأ�һ����ԣ�Ϊd0��d10�Ų�ʱ������ɫ��Ϊd1��d9�Ų�ʱ������ɫ����[Co��H2O��6]2+�Էۺ�ɫ���ݴ��жϣ�[Mn��H2O��6]2+����ɫ����ޡ����С�����
��4��L��ԭ�Ӻ������ռ��9�������������һ��δ�ɶԵ��ӣ�L��Cl����Ԫ�ط��ţ���
��5����ͼ1��ʾ��H2O2�Ŀռ乹�ͣ�H2O2������ÿ����ԭ�Ӷ����ӻ���H2O2Ϊ���� ������ԡ��Ǽ��ԡ������ӣ�
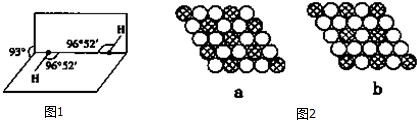
��6��H2S��H2O2����Ҫ�������������ʾ��
H2S��H2O2����Է�������������ͬ����������������ʲ������Ҫԭ����H2O2����֮����γ�������۷е�ߣ�H2O2��ˮ����֮����γ�������ܽ�ȴ�
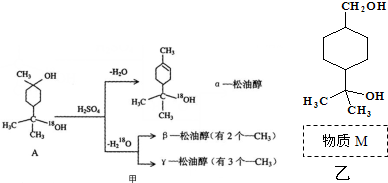
��7����ͼ2��ʾΪ��άƽ�澧��ʾ��ͼ������ʾ�Ļ�ѧʽΪAX3����b��
��1��C��Si��N�ĵ縺���ɴ�С��˳����N��C��Si��Na��Mg��Al�ĵ�һ�������ɴ�С��˳����Mg��Al��Na��
��2��A��B��Ϊ�����ڽ���Ԫ�أ����ݱ��е����ݣ�д��Bԭ�ӵĵ����Ų�ʽ��1s22s22p63s2��
| ������/��kJ•mol-1�� | I1 | I2 | I3 | I4 |
| A | 932 | 1��821 | 15��390 | 21��771 |
| B | 738 | 1��451 | 7��733 | 10��540 |
��4��L��ԭ�Ӻ������ռ��9�������������һ��δ�ɶԵ��ӣ�L��Cl����Ԫ�ط��ţ���
��5����ͼ1��ʾ��H2O2�Ŀռ乹�ͣ�H2O2������ÿ����ԭ�Ӷ����ӻ���H2O2Ϊ���� ������ԡ��Ǽ��ԡ������ӣ�

��6��H2S��H2O2����Ҫ�������������ʾ��
| �۵�/K | �е�/K | ˮ���ܽ�ȣ������ | |
| H2S | 187 | 202 | 2.6 |
| H2O2 | 272 | 423 | ������Ȼ��� |
��7����ͼ2��ʾΪ��άƽ�澧��ʾ��ͼ������ʾ�Ļ�ѧʽΪAX3����b��
 ��
��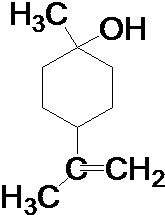 ��
��
 ��
�� ��
��