��Ŀ����
7��ijУ����С��ģ�ҵ�Ʊ�����ⶨ����Ĵ��ȣ��ס�������ͬѧ�ֱ�������������ʵ�飮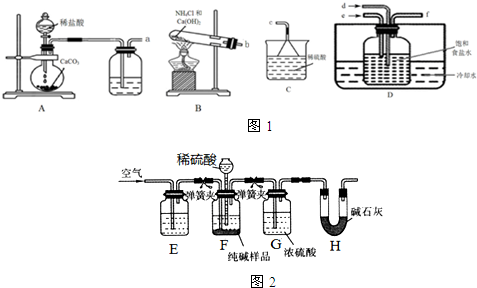
��1����֪̼���������ȷֽ�����̼���ơ�ˮ��CO2����̼�������Ʊ�����Ĺ������õ�����Ҫ�������ƾ��ơ������ǡ����żܡ��������⣬��������������ǯ��
��2������ͬѧ����ͼ1װ���Ʊ�̼�����ƣ�
��ͼ��װ�õ����ӷ���Ϊa��d��b��e��f��c��
��װ��D���Լ�ƿ�ڷ����Ļ�ѧ��Ӧ����ʽΪNaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
��ʵ����Ҫ��ͨ���NH3����֮����ͨ��CO2���壬����ͨ���NH3�ѹ�����ʵ���������պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ�����ܿ�f������ֽ������˵��������������
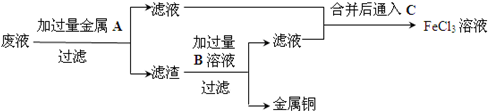
��3����֪ʵ���еõ���Na2CO3�г���������NaCl�����������ͼ2��ʾװ�����ⶨNa2CO3�ĺ�����
�ټ���װ��F�����Եķ����ǣ�����������©���������������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ�����ƿ�ڵ�ˮ�棬ֹͣ��ˮ���ã���Һ�治�½���˵��װ�ò�©����
��װ��E�е��Լ�NaOH��װ��G�����ø���CO2��
������ʵ��װ�ô�������ȱ�ݣ���ȱ�ݵ��²ⶨ���ƫ�ߣ���ȱ��Ϊװ��H��ȱ��ʢ�м�ʯ�ҵĸ���װ�ã�
���� ��1�����Ȼ�Ϲ�����Ҫ�������н��У�����Ҫ������ǯȡ��������
��2���ٰ�����������ˮ����Ҫ��ֹ����������ͨ�백���ĵ���Ӧ�����ԽӴ�Һ�棬��b����e��
�ڸ��ݲ������̣�����ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƺ�NH4Cl��
�۸��ݼ��鰱���IJ��÷������
��3���ټ���װ��F�����Եķ����ǣ�����������©���������������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ�����ƿ�ڵ�ˮ�棬ֹͣ��ˮ������ѹǿ�仯��Һ��仯�����жϣ�
��ʵ��װ���л�������ֶ�����̼��Ӱ��H�ж�����̼�����IJⶨ����Ҫͨ�������װ���ڵĶ�����̼��ȫ����H�б����գ������к��ж�����̼����Ӧ��������������Һ���գ���ֹӰ�������̼�IJⶨ��Ũ���������ˮ�ԣ��ܹ����ն�����̼�е�ˮ�֣�
��H��ȱ�����տ����ж�����̼��ˮ��װ�ã�������еĶ�����̼��ˮ������װ��H���գ�������������Na2CO3�ĺ�����ƫ�ߣ�
��� �⣺��1����̼�������Ʊ�����Ĺ����У���Ҫ����Ϲ�����������м��ȣ������õ�����Ҫ�������ƾ��ơ������ǡ����żܡ��������⣬��ȱ������������ǯ��
�ʴ�Ϊ������������ǯ��
��2���ٲ������̣�����ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƣ�������������ˮ��Ӧ�ý�D��ͨ��������ĩ�˸ոսӴ�Һ�棬����
a��d��b��e��f��c��
�ʴ�Ϊ��d��e��
������ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƺ�NH4Cl����Ӧ�Ļ�ѧ����ʽΪ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
�ʴ�Ϊ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
�ۼ��鰱��ͨ�����õķ����У�պ��Ũ����IJ������������а������ɣ���������ʪ��ĺ�ɫʯ������ֽ���������Ը�ʵ���м��鷽��Ϊ����պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ�����ܿ�f������ֽ������˵��������������
�ʴ�Ϊ����պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ�����ܿ�f������ֽ������˵��������������
��3���ټ���װ��F�����Եķ���Ϊ������������©���������������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ�����ƿ�ڵ�ˮ�棬ֹͣ��ˮ����©�������Լ�ƿ�е�Һ���ֲ��ٱ仯��֤��װ����������ã�
�ʴ�Ϊ��Һ�治�½���
��ʵ��װ���л�������ֶ�����̼��Ӱ��H�ж�����̼�����IJⶨ����Ҫͨ�������װ���ڵĶ�����̼��ȫ����H�б����գ������к��ж�����̼����Ӧ��������������Һ���գ���ֹӰ�������̼�IJⶨ����װ��F�����Ķ�����̼�����л���ˮ��Ӱ��ⶨ�������Ҫ��װ��G���������̼����С�ⶨ��
�ʴ�Ϊ��NaOH��Һ������CO2��
��װ��Hֱ�������������������еĶ�����̼��ˮ������װ��H���գ�����Hװ�õij����������ⶨ��Na2CO3�ĺ�����ƫ�ߣ����Ը�װ�õ�ȱ����װ��H��ȱ��ʢ�м�ʯ�ҵĸ���װ�ã�
�ʴ�Ϊ��װ��H��ȱ��ʢ�м�ʯ�ҵĸ���װ�ã�
���� ���⿼���˴��ҵ������ԭ������Ӧ�ã���Ŀ�Ѷ��еȣ���ȷʵ��Ŀ�ļ���ѧʵ�������������Ϊ���ؼ�������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ�����������������ѧʵ��������

��Na��ˮ��Ӧʱ������ˮ������
��������������Ӧ��ʵ��ʱ������Ƭ��Ϊ����
��Zn��ϡH2SO4��Ӧʽ���μ�����CuSO4��Һ
�ܳ�����Fe��ϡ���ᷴӦ��ȡ����ʱ������Ũ����
����H2O2��ȡO2ʱ����������MnO2��
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �٢ܢ� | D�� | �ڢۢ� |
| A�� | ͨ��������ˮ�� | B�� | ��ȼ | ||
| C�� | ������ | D�� | ͨ����������KMnO4��Һ�� |
| A�� | ��3a+b��mol | B�� | ��3a+$\frac{b}{2}$��mol | C�� | ��3a+3p+$\frac{b}{2}$��mol | D�� | ��3a+$\frac{b}{2}$-3p��mol |
 ��ͼװ�õ��һ��ʱ�䣬��ij������0.32gCuʱ��I��������ҺpH�ֱ�Ϊ ����Һ�����������Ϊ100mL�ҵ��ǰ����Һ����仯��������ܽ���Բ��ƣ���������
��ͼװ�õ��һ��ʱ�䣬��ij������0.32gCuʱ��I��������ҺpH�ֱ�Ϊ ����Һ�����������Ϊ100mL�ҵ��ǰ����Һ����仯��������ܽ���Բ��ƣ���������| A�� | 13��7��1 | B�� | 12��7��2 | C�� | 1��7��13 | D�� | 7��13��1 |
| A�� | 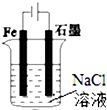 ͼ�������������ݲ������μӷ�̪��Һʱʯīһ����� | |
| B�� | 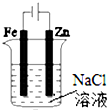 ͼ��װ�ÿ�����֤�������������������� | |
| C�� |  ͼ�ۿ���ģ�������������ʴ������һ���ĵ缫��Ӧʽ��Fe=Fe 2++2e- | |
| D�� | 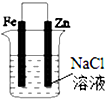 �ֱ������4��װ�õ����缫�����μ�����K3[Fe��CN��6]��Һ���ڢܳ�����ɫ���� |
| A�� | ����Ԫ��ԭ�ӵĺ�����Ӳ���֮��������������֮����ȣ��������ڱ���ǰ10��Ԫ���У�����������ϵ��Ԫ�ع���2�� | |
| B�� | ijԪ�����γ�+7�۵ĺ����ἰ���Σ����Ԫ��һ��Ϊ����Ԫ�� | |
| C�� | L�����Ϊż������������Ԫ����������������Ԫ��ԭ�ӵ�L���������� | |
| D�� | ͬ�������ֶ�����Ԫ��ԭ������֮�������2 |
 �Ҵ������в�ͬ�Ļ�ѧ����ͼ��ʾ�����Ҵ��ڸ��ַ�Ӧ��Ӧ���ѵļ�˵������ȷ���ǣ�������
�Ҵ������в�ͬ�Ļ�ѧ����ͼ��ʾ�����Ҵ��ڸ��ַ�Ӧ��Ӧ���ѵļ�˵������ȷ���ǣ�������| A�� | �����������ʱ�����ٶ� | |
| B�� | Ũ���Ṳ����170��ʱ�����ں͢ݶ��� | |
| C�� | �����ᡢŨ���Ṳ��ʱ�����ڶ��� | |
| D�� | �������º�������Ӧʱ�����ٺܶ͢��� |