��Ŀ����
����Ŀ��A��B��C��D��E��F���ֶ���������Ԫ�أ�ԭ������������������B�ĵ����ڳ�����Ϊ˫ԭ�ӷ��ӣ�����A�ĵ��ʿ��γɷ���X��X��ˮ��Һ�ʼ��ԣ�D�ļ���������X������ͬ����������D��ͬ�����м����Ӱ뾶��С��Ԫ�أ�EԪ�ص�ԭ�������ȴ�������������ӣ�C��F����Ԫ�ص�ԭ������㹲��13�����ӡ���
��1��A��Ԫ�ط���______________��D��Ԫ������ ____________��
��2��C�����ڱ��е�λ��Ϊ_______��E�����ӽṹʾ��ͼ________��
��3��B��C��E�ֱ���A�γɵĻ����������ȶ�����________(д��ѧʽ)��E��F������������Ӧ��ˮ��������Խ�ǿ����________(д��ѧʽ)
��4��X�ڴ�����C�����п�������ȼ�գ�����B�ĵ��ʡ��÷�Ӧ�Ļ�ѧ����ʽΪ��__________________��
���𰸡�H �� �� 2 ���� ��A��  H2O HClO4 4NH3+3O2(����)
H2O HClO4 4NH3+3O2(����)![]() 2N2+6H2O
2N2+6H2O
��������
A��B��C��D��E��F���ֶ���������Ԫ�أ�ԭ��������������������B�ĵ����ڳ�����Ϊ˫ԭ�ӷ���������A�ĵ��ʿ��γɷ���X��X��ˮ��Һ�ʼ�����A��ԭ��������BС����BΪNԪ����AΪHԪ����XΪNH3��D�ļ���������X������ͬ��������˵��D�ļ������Ӻ�����10��������D��ͬ�����м����Ӱ뾶��С��Ԫ�أ���DΪAlԪ�أ�EԪ�ص�ԭ�������ȴ�������������ӣ�EΪSԪ�أ���FΪClԪ�أ�C��F����Ԫ�ص�ԭ������㹲��13�����ӣ�˵��CԪ�ص��������6�����ӣ�CΪOԪ�ء�
(1). A����Ԫ�أ�Ԫ�ط���ΪH��D��AlԪ�أ�Ԫ������Ϊ�����ʴ�Ϊ��H������
(2). C��OԪ�أ���2�����Ӳ㣬�������6�����ӣ�����OԪ�������ڱ��е�λ���ǵ�2���ڵ�VIA�壬E��SԪ�أ�S2�������ӽṹʾ��ͼΪ��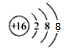 ���ʴ�Ϊ����2���ڵ�VIA����
���ʴ�Ϊ����2���ڵ�VIA���� ��
��
(3). �ǽ�����Խǿ����Ԫ�ض�Ӧ���⻯��Խ�ȶ���N��O��S����Ԫ���У��ǽ�����ǿ����OԪ�أ�����ˮ���ȶ����ǽ�����Խǿ����Ԫ�ص�����������Ӧ��ˮ��������Խǿ���ǽ�����Cl��S�������Խ�ǿ����HClO4���ʴ�Ϊ��H2O��HClO4��
(4). �����ڴ����������п�������ȼ�գ����ɵ�����ˮ�����ݵ�ʧ�����غ��ԭ���غ㣬�÷�Ӧ�Ļ�ѧ����ʽΪ��4NH3+3O2(����)![]() 2N2+6H2O���ʴ�Ϊ��4NH3+3O2(����)
2N2+6H2O���ʴ�Ϊ��4NH3+3O2(����)![]() 2N2+6H2O��
2N2+6H2O��

����Ŀ�������л���ķ���ʽ��ΪC8H10O

�ش���������:
(1)���������У���ʹ����KMnO4��Һ��ɫ��������_______(����ĸ)��
(2)D�����й�ƽ���̼ԭ����______����
(3)C��ʹBr2��CCl4��Һ��ɫ���÷�Ӧ�ķ�Ӧ��������_______________��
(4)��֪:
���� | �е�/�� | �۵�/�� | �ܽ��� |
A | 176.7 | -32.1 | ������ˮ�����봼���ѵȶ����л��ܼ����� |
D | 217 | 61.5 | ���ڴ����ѡ��ȷ£�����ˮ |
����A��D�Ļ�������ͨ��_____(��ʵ���������)���з��롣
����Na��Fe���ֽ���������ʵ���ҿ�����������A��D����____,д�����е�ʵ�鷽����___��
����Ŀ��(1)��һ�ֿɳ����Na-Al/FeS����ع���ʱNa+�����ʵ������ֲ��䣬�������ú�Na+�ĵ��������Ϊ����ʣ���֪�õ��������ӦʽΪ2Na++FeS+2e-=Na2S+Fe����õ���ڳ��ʱ,�����ĵ缫��Ӧʽ_________________�ŵ�ʱ������Ӧ��������__________________��
(2)��ͼ��ʾ�������ݻ�Ϊ���ҵ��������¶���ͬ���ֱַ�����ͼ��ʾ�����������壬ͬʱ���������������ʹ�����������ַ�Ӧ�ﵽƽ�⣬������������Ӧ�ٴδﵽƽ�⣬����˵����ȷ����__________
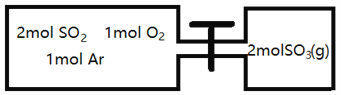
A����һ��ƽ��ʱ��SO2�����ʵ������Ҹ���
B����һ��ƽ��ʱ��������ѹǿһ��С��ʯ��
C���ڶ���ƽ��ʱ��SO3�����ʵ��������ȵ�һ��ƽ��ʱ���ҵ�SO3�����ʵ���������
D���ڶ���ƽ��ʱ��SO2�������ʵ����ȵ�һ��ƽ��ʱ����SO2���ʵ�����2��С
(3)��֪Ksp (Ag2CrO4)=1.0��10-12����0.02mol/L��AgNO3��Һ�м���������1. 0��10-4mol/LK2CrO4��Һ������Һ��c(CrO42-)=__________
(4)�����£�0.1mol/LNaHCO3��Һ��pHֵ__________0.1mol/LNa2SO3��Һ��pHֵ(�>������<������=��)��֪��
H2CO3 | K1=4.3��10-7 | K2=5.6��10-11 |
H2SO3 | K1=1.54��10-2 | K2=1.02��10-7 |
�����£���0.5mol/LNa2SO3��Һ�м�������ˮ����ˮ�������c(H+) ��c(OH-)__________��(��������С���������䡱)
