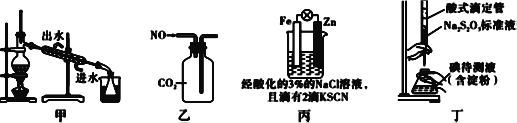
��Ŀ����
����Ŀ����̼����(Na2CO4)��һ�ֺܺõĹ�����������ϡ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ��2Na2CO4+4HCl=4NaCl+2CO2��+O2��+2H2O�����۹�̼����һ�㶼����̼���ƣ�Ϊ�ⶨij��̼������Ʒ(ֻ��Na2CO4��Na2CO3)�Ĵ��ȣ�ij��ѧ��ȤС������������ַ���ʵʩ��
����һ��

(1)�����ٺ͢۵����Ʒֱ�Ϊ_______��________��
(2)���������У�ʹ�õ�����������________(��������)��
(3)����������۵IJ�������___________��
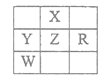
������������ͼ��װ��ʵ��װ�ã�QΪһ�ɹ��͵�����������ȡ������Ʒ�����У���Һ©����������ϡ�����������������ַ�Ӧ

(4)Ϊ�ⶨ��Ӧ������������������ϡ����ǰ����ر�____��____ (���K1������K2����K3��)������A��������________��
(5)��������Ӧֹͣ��ʹK1��K3���ڹر�״̬��K2���ڴ�״̬���ٻ�����K1��B��װ�Ĺ����Լ���_________��ΪʲôҪ������K1?_______________��
(6)ʵ�����ʱ����Ͳ1����xmLˮ����ͲII���ռ�����ymL���壬����Ʒ�й�̼���Ƶ�����������________(�ú���x��y�Ĵ���ʽ��ʾ)
���𰸡� ���� �����ᾧ �ڢ� �����������������г��ִ�������ʱ��ֹͣ���ȣ�Ȼ���������������������еĹ��� K1��K2 K3 ƽ���Һ©���ںͷ�Ӧ��ϵ��ѹǿ��ʹϡ����˳�����£�ͬʱ�������µ�ϡ������������������Ӱ�� ��ʯ��(���������𰸾�����) �����ɵ�CO2�ܳ�ֱ�B�м�ʯ�����գ�ʹ��Ͳ���ռ����ϴ�ںO2 l22y/(53x-37y)��100%
������������ʵ�鷽����������ۣ���1�����ݷ���һ��������Ϊ������������ӦΪ�����ᾧ����2����������ʹ�ò��������������������ǽ��裬���ٷ�Ӧ������������Ҫ���������������������ǽ��裬��ֹ���Ȳ��������Һ��ɽ�����3���������������ᾧ�����������Ǽ����������������г��ִ�������ʱ��ֹͣ���ȣ�Ȼ���������������������еĹ��壻��4����ʵ���Ŀ���Dzⶨ��Ӧ������������������ͲI�Dz��������������װ�ã����ζ�ϡ����ǰ�ر�K1��K2����K3������A�������Ǽ����������������г��ִ�������ʱ��ֹͣ���ȣ�Ȼ���������������������еĹ��壻��5��װ��II�е���Ͳ�ռ��������壬��B������������CO2��B��ʢ�ż�ʯ�һ��������ƹ���ȣ�������K1��Ŀ���������ɵ�CO2�ܳ�ֱ�B���գ�ʹ��Ͳ���յ��Ľϴ�����O2����6����ͲI�вⶨ����CO2��O2�������ͲII�ⶨ��������������ݹ�̼���������ᷴӦ����ʽ�������̼���Ƶ�����Ϊ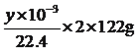 ��CO2�����Ϊ(x��y)mL����̼���Ʋ���CO2�����Ϊ2ymL��̼���Ʋ���CO2�����Ϊ(x��3y)mL����̼���Ƶ�����Ϊ
��CO2�����Ϊ(x��y)mL����̼���Ʋ���CO2�����Ϊ2ymL��̼���Ʋ���CO2�����Ϊ(x��3y)mL����̼���Ƶ�����Ϊ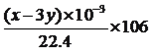 g����̼���Ƶ���������Ϊ
g����̼���Ƶ���������Ϊ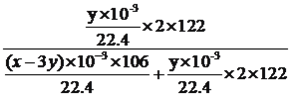 ��100%=l22y/(53x-37y)��100%��
��100%=l22y/(53x-37y)��100%��

����Ŀ�������¶��£�������������ĺ����ܱ������У���Ӧ2CO2(g)+6H2(g)![]() C2H5OH(g)+3H2O(g)��ƽ�⣬����˵����ȷ����( )
C2H5OH(g)+3H2O(g)��ƽ�⣬����˵����ȷ����( )
���� | �¶�/K | ���ʵ���ʼŨ��(mol/L) | ���ʵ�ƽ��Ũ��(mol/L) | |||
CO2(g) | H2(g) | C2H5OH(g) | H2O(g) | C2H5OH(g) | ||
�� | 500 | 0.20 | 0.60 | 0 | 0 | 0.083 |
�� | 500 | 0.40 | 1.20 | 0 | 0 | |
�� | 600 | 0 | 0 | 0.10 | 0.30 | 0.039 |
A. �÷�Ӧ����ӦΪ���ȷ�Ӧ
B. ��ƽ��ʱ���ס��������ڣ�2c(CO2����)<c(CO2����)
C. ��ƽ��ʱ���������е��淴Ӧ���ʱ��������еĴ�
D. ��ƽ��ʱ��ת���ʣ�a(CO2����)+a(C2H5OH����)>1