��Ŀ����
����Ŀ������������ȼ�ϵ��Ϊ��Դ���е���ʵ��װ����ͼ��ʾ������˵����ȷ����( )
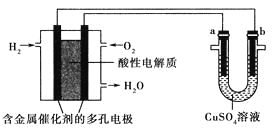
A. ȼ�ϵ�ع���ʱ��������ӦΪO2��2H2O��4e��=4OH��
B. ��⾫��ͭʱ����ת��1mol���ӣ�a����������32g
C. �������ͭʱ��aΪ����bΪCu������һ��ʱ��Ҫʹ�ҳ���Һ��ԭ�ɼ���������CuO
D. ��a��b������Ϊʯīʱ������ͬ�����£�a���������������������ĵ�O2�����ͬ
���𰸡�D
�����������������A�����ȼ�ϵ���������Ե�����й���������������ӦΪ��O2+4e-+4H+=2H2O����A����B����⾫��ͭʱ��a���Ǵ�ͭ���ܽ����ͭ�ͱ�ͭ���õĽ�������B����C���������ͭʱ������aΪͭ��bΪ��������һ��ʱ������ͭ��Ũ�Ȼ������䣬��C����D��a��b��������ʯīʱ�����CuSO4��Һʱ��a������������ΪO2������1molO2��4mol���ӣ�����ȼ�ϵ����Ҫ����1molO2�����ߵ������ȣ���D��ȷ����ѡD��

 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
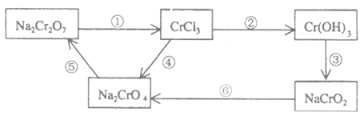
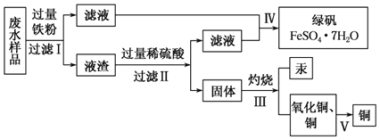
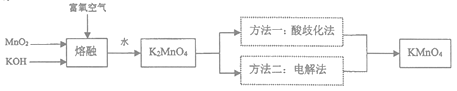
ѧϰʵ����ϵ�д�����Ŀ��KMnO4��һ����Ҫ�����������㷺���ڻ�ѧ�����ͻ��������Է�ˮ������ҵ����ҵ�Ͽ������̿�(��Ҫ�ɷ�ΪMnO2)�Ʊ���Ŀǰ�����ֽ�Ϊ������Ʒ�����ģ����������ͼ��ʾ��
��������ͬ�¶������ɳ����صĻ�������ܽ�ȣ���λ��g/(100gH2O)��
��ѧʽ | 20��C | 30��C | 40��C | 60��C | 80��C | 100��C |
CH3COOK | 256 | 283 | 324 | 350 | 381 | |
K2SO4 | 11.1 | 13 | 14.8 | 18.2 | 21.4 | 24.1 |
KCl | 34.2 | 37.2 | 40.1 | 45.8 | 51.3 | 56.3 |
KMnO4 | 6.34 | 9.03 | 12.6 | 22.1 | ||
K2CO3 | 111 | 114 | 117 | 127 | 140 | 156 |
(1)�����ڡ�ʱ��������������ʵ���________________(�����)��
A.������ B.�մ� C.����þ D.ʯӢ
(2)д��MnO2��KOH��������ͨ�븻������ʱ���K2MnO4�Ļ�ѧ����ʽ______________________��
(3)�����绯��������pH<6�������K2MnO4����ת��ΪMnO2��KMnO4�����˳�ȥMnO2������Һ��������Ũ�������ȹ��˵õ�KMnO4�־��壬�پ����ؽᾧ��ýϴ�����KMnO4���壻
�ٸ��ݱ��е��ܽ�������Լ������������ص㣬�����绯����������ѡ�������������________��
A.ϡ���� B.���� C.ϡ���� D.������̼
�ڡ�����Ũ����ʱ���¶��������70�棬���˵ļ��ȷ�ʽ��________________��
�۸�����ط���ʽ�����㡰���绯���������۲���Ϊ_________________��
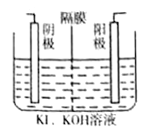
(4)����ⷨ���˷��ˡ����绯�������۲���ƫ�͵����⣬ͬʱ����ƷKOH���������̿�ı��ա���ⷨ�Ʊ�������ص�ʵ��װ��ʾ��ͼ����(ͼ�������ӽ���Ĥֻ����K+����ͨ��)
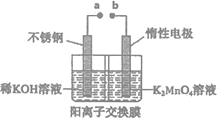
��aΪ______��(���������)�����ҷ����ĵ缫��Ӧ����ʽΪ_______________��
������ʼʱ��������ҺΪ1.0L0.40mol��L-1K2MnO4��Һ�����һ��ʱ���������n(K)/m(Mn)Ϊ6��5������������KOH������Ϊ_________________��