��Ŀ����
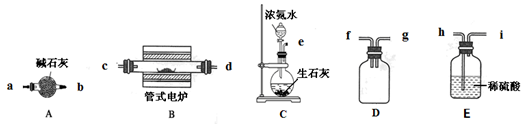
��14�֣�ij��ѧ��Ȥ̽��С�齫һ����������·������õ���Cu��70������Al��25������Fe��4����������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�

��1���ڽ������������ķ�Ӧ�У����������Ե�����Ϊ ���õ�����1����Ҫ�ɷ�Ϊ ��
��2���ڢڲ���H2O2������Ӧ�����ӷ���ʽΪ ��
ͨ������NaOH������Һ��pH����Ŀ���� ��
��3���������а�����ʵ�鲽���� �����ˣ�
��4��ͨ�����������������2��ȡAl2(SO4)3��18H2O �������Dz������һ��ʵ�鷽����
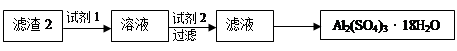
ʵ���У��Լ�1�� ���Լ�2�� �����ѧʽ��

��1���ڽ������������ķ�Ӧ�У����������Ե�����Ϊ ���õ�����1����Ҫ�ɷ�Ϊ ��
��2���ڢڲ���H2O2������Ӧ�����ӷ���ʽΪ ��
ͨ������NaOH������Һ��pH����Ŀ���� ��
��3���������а�����ʵ�鲽���� �����ˣ�
��4��ͨ�����������������2��ȡAl2(SO4)3��18H2O �������Dz������һ��ʵ�鷽����
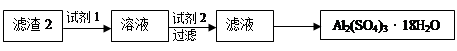
ʵ���У��Լ�1�� ���Լ�2�� �����ѧʽ��
19����14�֣�
��NO3- Au��Pt ��2Fe2++H2O2+2H+ = 2Fe3++2H2O ʹAl3+ ��Fe3+ת��Ϊ����
����������ȴ�ᾧ ��H2SO4 Al ��ÿ��2�֣�
��NO3- Au��Pt ��2Fe2++H2O2+2H+ = 2Fe3++2H2O ʹAl3+ ��Fe3+ת��Ϊ����
����������ȴ�ᾧ ��H2SO4 Al ��ÿ��2�֣�
��������������Ի�������������Ӿ��������ԣ�ֻ��Au��Pt����������ͼ����������Ҫ�ɷ���Au��Pt��������������ܽ���Һ�е�����������ȫ����Ϊ�����ӣ������������Ƶ�����Һ��pH��ʹ�����Ӻ���������ȫ���������������Һ2Ϊ����ͭ��Һ���������ᾧ�Ϳ��Եõ�����ͭ���壬����2�к��������������������������������������ʹ����ȫ�ܽ⣬�ټ����������û����������е����������������ʺ���������
�����������Ѷ��еȣ���Ҫ�����˳������������ʼ�һЩ��ѧʵ���������������ʵ��Ŀ��飬����Ҫ���������⣬Ȼ��۲�ʵ��װ�ã�������ÿһ��ʵ��װ�õ������ͼ���ٽ��ʵ�ʵĻ�ѧ��Ӧ������������Ʋ⡣

��ϰ��ϵ�д�
�����Ŀ
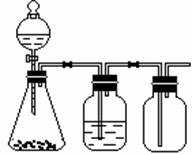
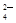 �Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽������������(���ڳ������Լ��Թ���)��
�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽������������(���ڳ������Լ��Թ���)��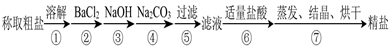
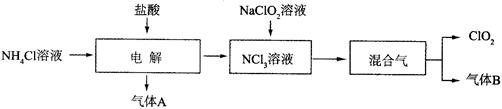
 2BN + 3H2O������֪��BN�������ܱ�����������������ˮ��B2O3��������ˮ����������ˮ����
2BN + 3H2O������֪��BN�������ܱ�����������������ˮ��B2O3��������ˮ����������ˮ����