��Ŀ����
9��ij�о�С���Դ��κ�̼����泥�NH4HCO3��Ϊԭ�ϣ�������ͼ�����Ʊ�������Ȼ�泥���֪�ε��ȷֽ��¶ȣ�NH4HCO3 36�棻NaHCO3 270�棻NH4Cl 340�棻Na2CO3��850��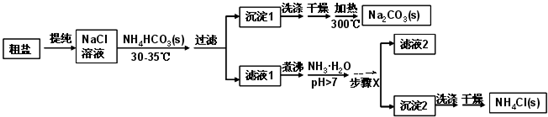
��1�������к���Ca2+��Mg2+��SO42-���������ӣ�ʹ�õ��Լ��У���NaOH ��BaCl2��HCl ��Na2CO3��������˳���������B
A���٢ۢܢ�B���٢ڢܢ�C���ۢڢܢ�D���٢ڢۢ�
�������õ���������������������
��2������жϴ�����SO42-�Ƿ��ѳ�����ȡ�������Ӻ����Ʒ���Թ��У��μ������Ȼ�����Һ�����ް�ɫ������������SO42-�ѳ�����
��3����NaCl��Һ������1�Ĺ����У�������Ũ�����ڼ������NH4HCO3֮ǰ��������Ũ�������ڼ���NH4HCO3֮��ԭ���ǿɱ���NH4HCO3�ķֽ⣻
��4��д������1���ȷֽ�Ļ�ѧ����ʽ2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2����
��5��Ϊ���NH4Cl��Ʒ�IJ��ʺʹ��ȣ�������Һ�м��백ˮ������������NH4+ˮ�⡢ʹNaHCO3ת��ΪNa2CO3���������ʱ��ʧ��NH3������X�����IJ���������Ũ������ȴ�ᾧ�����ˣ�
��6�����á���ȩ�����ⶨ��ҵ��Ʒ���Ȼ�淋Ĵ��ȣ��ٶ����ʲ����ȩ��Ӧ������Ӧԭ��Ϊ��4NH4Cl+6HCHO����CH2��6N4+4HCl+6H2O ij������Ա��ȡ1.5g����Ʒ����ˮ�����������ļ�ȩ����ˮ���100mL��Һ������ȡ��10mL�������̪����0.1mol/L��NaOH��Һ�ζ������ζ��յ�ʱ����NaOH��Һ25.00mL�������Ʒ�� �Ȼ�淋���������Ϊ89.2%��
���� �����������̿�֪������ˮ�ᴿ����Ȼ�����Һ��������Ũ�����¶ȿ�����30�桫35�棬��ֹ̼���ⰴ�ֽ⣬����̼����泥����ɳ���1Ϊ̼�����ƣ���Һ1��ҪΪ�Ȼ�泥��Ȼ����Һ�м��백ˮ��������笠����ӵ�ˮ�⣬��������Ũ������ȴ�ᾧ�����˿ɵó���2Ϊ�Ȼ�粒��壬��ϴ�ӡ�����ô������Ȼ�泥���Һ2�к����������Ȼ�狀�̼�����ƣ�
��1�������к���Ca2+��Mg2+��SO42-���������ӣ�ʹ�õ��Լ��У���NaOH ��BaCl2��HCl ��Na2CO3������ǰ�����������ǹ����ģ��ں������������ڳ�ȥ��Һ��ԭ���������Ҫ��ǰ�������Լ����ʳ�ȥ���ݴ��жϣ��������õ���������������������
��2����Ʒ�м����������Ȼ�����Һ���жϴ�����SO42-�Ƿ��ѳ�����
��3������Ũ��ʱ��ҺҪ���ȣ���NH4HCO3��36�濪ʼ�ֽ⣬�ݴ˴��⣻
��4��̼���������ȷֽ�����̼���ơ�������̼��ˮ��
��5����ˮ������笠����ӵ�ˮ�⣬ͬʱ��ʹNaHCO3ת��ΪNa2CO3�����������ʱ��ʧ��NH3�����Ȼ����Һ�õ��Ȼ�粒������ͨ������Ũ������ȴ�ᾧ�����˵ķ�����
��6���ɵζ���ȥ���������Ƶ����ʵ����������������ʵ��������ݷ���ʽ4NH4Cl+6HCHO����CH2��6N4+4HCl+6H2O��������Ȼ�淋������������������Ʒ���Ȼ�淋�����������
��� �⣺�����������̿�֪������ˮ�ᴿ����Ȼ�����Һ��������Ũ�����¶ȿ�����30�桫35�棬��ֹ̼���ⰴ�ֽ⣬����̼����泥����ɳ���1Ϊ̼�����ƣ���Һ1��ҪΪ�Ȼ�泥��Ȼ����Һ�м��백ˮ��������笠����ӵ�ˮ�⣬��������Ũ������ȴ�ᾧ�����˿ɵó���2Ϊ�Ȼ�粒��壬��ϴ�ӡ�����ô������Ȼ�泥���Һ2�к����������Ȼ�狀�̼�����ƣ�
��1�������к���Ca2+��Mg2+��SO42-���������ӣ�ʹ�õ��Լ��У���NaOH ��BaCl2��HCl ��Na2CO3������ǰ�����������ǹ����ģ��ں������������ڳ�ȥ��Һ��ԭ���������Ҫ��ǰ�������Լ����ʳ�ȥ�����Լ����˳��ΪNaOH��BaCl2��Na2CO3��HCl����ѡB���������õ���������������������
�ʴ�Ϊ��B��������
��2����Ʒ�м����������Ȼ�����Һ���жϴ�����SO42-�Ƿ��ѳ������������Ϊȡ�������Ӻ����Ʒ���Թ��У��μ������Ȼ�����Һ�����ް�ɫ������������SO42-�ѳ�����
�ʴ�Ϊ��ȡ�������Ӻ����Ʒ���Թ��У��μ������Ȼ�����Һ�����ް�ɫ������������SO42-�ѳ�����
��3������Ũ��ʱ��ҺҪ���ȣ���NH4HCO3��36�濪ʼ�ֽ⣬�����ڼ������NH4HCO3֮ǰ��������Ũ�������ڼ���NH4HCO3֮��ԭ���ǿɱ���NH4HCO3�ķֽ⣬
�ʴ�Ϊ���ɱ���NH4HCO3�ķֽ⣻
��4��̼���������ȷֽ�����̼���ơ�������̼��ˮ����Ӧ����ʽΪ2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2����
�ʴ�Ϊ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2����
��5����ˮ������笠����ӵ�ˮ�⣬ͬʱ��ʹNaHCO3ת��ΪNa2CO3�����������ʱ��ʧ��NH3�����Ȼ����Һ�õ��Ȼ�粒������ͨ������Ũ������ȴ�ᾧ�����˵ķ�����
�ʴ�Ϊ������NH4+ˮ�⡢ʹNaHCO3ת��ΪNa2CO3���������ʱ��ʧ��NH3������Ũ������ȴ�ᾧ�����ˣ�
��6���������⣬�ζ���ȥ���������Ƶ����ʵ���Ϊ0.025L��0.1mol/L=0.0025mol��������������ʵ���Ϊ0.0025mol�����ݷ���ʽ4NH4Cl+6HCHO����CH2��6N4+4HCl+6H2O����֪1.5g����Ʒ���Ȼ�淋�����Ϊ0.0025mol��$\frac{100}{10}$��53.5g/mol=1.3375g��������Ʒ���Ȼ�淋���������Ϊ$\frac{1.3375g}{1.5g}$��100%=89.2%��
�ʴ�Ϊ��89.2%��
���� ���⿼������Ƽ���ܽ�ȡ����ʵķ����ᴿ�����ʺ����ⶨ�ȣ��Ѷ��еȣ����ؿ���ѧ�������̵ķ����ͻ���֪ʶ������Ӧ�ã�

 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�| A�� | �������ƿ�����ʳƷ����ë��֯��ȵ�Ư�� | |
| B�� | ��������Ʒ����ֱ�Ӵ�������������� | |
| C�� | ȼ���̻���������ijЩ����Ԫ�ص���ɫ��Ӧ | |
| D�� | ���������ã�������Ʒ������Ʒ�ڿ�������ʴ |
| ʵ �� ˳ �� | ʵ �� �� �� | ʵ �� �� �� |
| �� | A+B | û������ |
| �� | B+D | ������ų� |
| �� | B+C | ������� |
| �� | A+D | ������� |
��2��д������ʵ���е��йػ�ѧ����ʽ��
��HCl+AgNO3�TAgCl��+HNO3��
��CaCl2+Na2CO3�T2NaCl+CaCO3����
| A�� | V=2.24L | |
| B�� | �μӷ�Ӧ��HClΪ0.18mol | |
| C�� | ��ȷ���������ɺ��ػ�����������Ƕ��� | |
| D�� | 6.32g KMnO4��ĩ��ֱ��������Ũ���ᷴӦ�ɵõ�2.24L Cl2 |
| A�� | a=10��b=9.2 | B�� | a��10��b��9.2 | C�� | a��10��b=9.2 | D�� | a��10��b��9.2 |
 ����ҵ�ϣ��ñ���ʯ�����ۼ���ʯī���缫���������������������д����ⷴӦ����ʽ2Al2O3$\frac{\underline{\;���\;}}{\;}$4Al+3O2�����ڵ����������������������������Ҫ���ڲ��䣮
����ҵ�ϣ��ñ���ʯ�����ۼ���ʯī���缫���������������������д����ⷴӦ����ʽ2Al2O3$\frac{\underline{\;���\;}}{\;}$4Al+3O2�����ڵ����������������������������Ҫ���ڲ��䣮