��Ŀ����
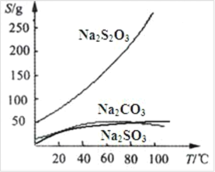
����Ŀ����ѧ���о���һ������Ȼ��Ϊȼ�ϵġ�ȼ��ǰ����ϵͳ��,���������ͼ��ʾ����(����������δ���).

��1����ҵ�Ͽ���H2��CO2�Ʊ��״�,�䷴ӦΪ��CO2(g)+3H2(g)CH3OH(g)+H2O(g),ij�¶���,��1mol CO2��3mol H2������������2L�ܱ������У�����������Ӧ����ò�ͬʱ�̷�Ӧǰ���ѹǿ��ϵ�����
ʱ��/h | 1 | 2 | 3 | 4 | 5 | 6 |
p��/pǰ | 0. 90 | 0. 85 | 0. 83 | 0. 81 | 0. 80 | 0. 80 |
����H2��ʾǰ2hƽ����Ӧ����v(H2)=___.
�ڸ��¶���CO2��ƽ��ת����Ϊ___.
��2����300�桢8MPa�£���������̼�����������ʵ���֮��Ϊ1:3ͨ��һ��ѹ�ܱ������з���(1)�з�Ӧ���ﵽƽ��ʱ����ö�����̼��ƽ��ת����Ϊ50%����÷�Ӧ�����µ�ƽ�ⳣ��Kp=______________(��ƽ���ѹ����ƽ��Ũ�ȼ���,��ѹ=��ѹ�����ʵ�������).
��3�������¶Ⱥ�ѹǿ�£���2 L�ĺ����ܱ������кϳɰ���:N2(g)+3H2(g)![]() 2NH3(g) ��H=-92. 4 kJ mol-1���ڷ�Ӧ�����з�Ӧ�������������ʵ�����ʱ��ı仯��ͼ��ʾ��
2NH3(g) ��H=-92. 4 kJ mol-1���ڷ�Ӧ�����з�Ӧ�������������ʵ�����ʱ��ı仯��ͼ��ʾ��

����10��20 min�ڣ�NH3Ũ�ȱ仯��ԭ�������_______��
A. ������� B. ��С������� C. �����¶� D. ����NH3�����ʵ���
�� 20 min�ﵽ��һ��ƽ�⣬�ڷ�Ӧ������25 minʱ�����߷����仯��ԭ����____________��35min�ﵽ�ڶ���ƽ�⣬��ƽ���ƽ�ⳣ��K1______K2������>����<������ = ����
���𰸡�0. 225mol/��Lh�� 40% ![]() AB ���� 0. 1 molNH3 =
AB ���� 0. 1 molNH3 =
��������
��1������������ʽ��������![]() =
=![]() ��������ʵı仯�����ټ��������ķ�Ӧ���ʣ�
��������ʵı仯�����ټ��������ķ�Ӧ���ʣ�
����������ʽ��������![]() =
=![]() ��������ʵı仯�����ټ��������̼ת���ʣ�
��������ʵı仯�����ټ��������̼ת���ʣ�
��2����������ʽ��������![]() =
=![]() ��������ʵı仯�����ټ�������ʵ����ʵ���������������ƽ���ѹ������
��������ʵı仯�����ټ�������ʵ����ʵ���������������ƽ���ѹ������
��3������ͼ���֪��������ʵ����仯���ӣ���10minʱ�仯�������ģ�˵��10minʱ�ı�������ʹ�����淴Ӧ���ʾ�����
����ͼ���֪����25���ӣ�NH3�����ʵ�������0. 1 mol��ƽ�ⳣ�����¶ȱ仯���¶Ȳ����ʱƽ�ⳣ��K�����䡣
��1������2hʱCO2��������Ϊx�������⽨����������ʽ��
CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g),
CH3OH(g)+H2O(g),
��mol�� 1 3 0 0
�䣨mol�� x 3x x x
ƽ��mol�� 1��x 3��3x x x
��![]() =
=![]() �ɵã�
�ɵã�![]() =
=![]() ��x=0.3mol����ǰ2hƽ����Ӧ����v(H2)=
��x=0.3mol����ǰ2hƽ����Ӧ����v(H2)=![]() 0. 225mol/��Lh�����ʴ�Ϊ��0. 225mol/��Lh����
0. 225mol/��Lh�����ʴ�Ϊ��0. 225mol/��Lh����
��������������ݿ�֪5hʱ����Ӧ�ﵽƽ�⣬���¶���CO2��ƽ��ת����Ϊa�������⽨����������ʽ��
CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g),
CH3OH(g)+H2O(g),
��mol�� 1 3 0 0
�䣨mol�� a 3a a a
ƽ��mol�� 1��a 3��3a a a
��![]() =
=![]() �ɵã�
�ɵã�![]() =
=![]() ��x=0.4mol����CO2��ƽ��ת����Ϊ
��x=0.4mol����CO2��ƽ��ת����Ϊ![]() ��100%=40%���ʴ�Ϊ��40%��
��100%=40%���ʴ�Ϊ��40%��
��2������ʼ������̼�����������ʵ����ֱ�Ϊ1mol��3mol�������⽨����������ʽ��
CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g),
CH3OH(g)+H2O(g),
��mol�� 1 3 0 0
�䣨mol�� 0.5 1.5 0.5 0.5
ƽ��mol�� 0.5 1.5 0.5 0.5
ƽ��ʱ������̼���״���ˮ�����ʵ�������Ϊ![]() ��ƽ���ѹΪ
��ƽ���ѹΪ![]() ��8MPa�������ĵ����ʵ�������Ϊ
��8MPa�������ĵ����ʵ�������Ϊ![]() ��ƽ���ѹΪ
��ƽ���ѹΪ![]() ��8MPa����Kp=
��8MPa����Kp=![]() =
=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��3������ͼ���֪��������ʵ����仯���ӣ���10minʱ�仯�������ģ�˵��10minʱ�ı�������ʹ�����淴Ӧ���ʾ�����
A��ʹ�ô����������淴Ӧ����������ȷ��
B����С����������൱������ѹǿ�������淴Ӧ���ʾ�������ȷ��
C�������¶ȣ���Ӧ���ʼ�С���ʴ���
D������NH3���ʵ�����10minʱNH3���ʵ���Ӧƫ��仯�㣬�ʴ���
AB��ȷ���ʴ�Ϊ��AB��
����ͼ���֪����25���ӣ�NH3�����ʵ�������0. 1 mol����H2��N2�����ʵ������䣬˵��Ӧ�Ƿ����0. 1 mol NH3������Ӧ���е�ʱ35-40min�������ʵ������䣬˵����Ӧ�ﵽ�ڶ���ƽ��״̬��ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬ƽ�ⳣ�����䣬�ʴ�Ϊ������0.1molNH3��=��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�