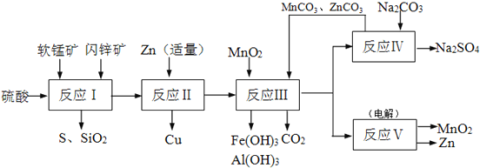
��Ŀ����
����Ŀ��(1)��H2O2��H2SO4 �Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��Cu(s) +2H+(aq) =Cu2+(aq) +H2(g) ��H=64kJ/mol��2H2O2(l)=2H2O(l)+O2(g) ��H= -196kJ/mol��H2(g)+1/2O2(g)=H2O(l) ��H= -286kJ/mol.��H2SO4��Һ��Cu��H2O2��Ӧ����Cu2+��H2O���Ȼ�ѧ����ʽΪ___________��
(2)��������������ͬ��ӡˢ��·��Ľ�����ĩ��10�GH2O2��3.0 mol/L��H2SO4�����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ�����(���±�)��
�� ��(��) | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
ͭƽ���ܽ�������10-3mol.L-1.min-1 | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ����______________��
(3)���ᴿ���CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ�����ȣ�����CuCl�������Ʊ�CuCl�����ӷ���ʽ��_________________________��
���𰸡���7�֣�
��1�� Cu(s)+H2O2(l)+2H��(aq)=Cu2��(aq)+2H2O(l) ��H=-320KJ.mol-1��3�֣�
��2��H2O2�ֽ����ʼӿ� ��2�֣�
��3��2Cu2��+SO32-+2Cl��+H2O![]() 2CuCl��+SO42-+2H����2�֣�
2CuCl��+SO42-+2H����2�֣�
��������
���⣨1�����ݸ�˹���ɵ�Ŀ�귽��ʽ=���١�5+��+�ۡ�2����1/2�����������Ȼ�ѧ����ʽΪCu(s)+H2O2(l)+2H��(aq)=Cu2��(aq)+2H2O(l) ��H=-320KJ.mol-1��
��2���¶����ߣ���������ֽ��Ҳ�죬������ͭ��Ӧ�Ĺ��������Ũ�ȼ�С����Ӧ���ʽ��ͣ�
��3��������Ŀ��Ϣ��CuSO4����������Na2SO3����ԭ��������������ԭ��Ӧ����CuCl�����������ƣ����ӷ���ʽΪ2Cu2��+SO32-+2Cl��+H2O![]() 2CuCl��+SO42-+2H����
2CuCl��+SO42-+2H����

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�