��Ŀ����
��13�֣���A��B��C��D��E��R���ֶ�����Ԫ�أ���Ԫ��������Ϣ���±���
�ش��������⣺
��1��д������Ԫ�ص����ƣ�C ��E ������B�������ӽṹʾ��ͼ�� ��
��2��B��C��D�ļ����Ӱ뾶�ɴ�С��˳���� ���û�ѧʽ��ʾ����EԪ�ص���ۺ�����Ļ�ѧʽ�� ��
��3��ʵ��֤��D�����������������������ԵIJ��������ǣ� ��
��4����һ�������£�RO32����R2���ɷ�����Ӧ��RO32����2R2����6H�� = 3R��3H2O�������й������У���ȷ���� ������ţ���
A��Ԫ��Rλ�����ڱ��е�3 ���ڣ���A��
B��R2������ԭ�ӵ��Ӳ�ṹ��ͬ
C��RO32�������ܷ��������з�Ӧ�ж�ֻ����������
D����Ӧ������1 mol RO32�� ��ת�Ƶĵ��ӵ����ʵ���Ϊ4 mol
��5����д���漰����Ԫ�ؼ��������Ӧ�����ӷ���ʽ��Ҫ��1������֮����û� ��2���ڷ���ʽ����Ϊ���ַǽ���������Dz�ͬ����ġ� �� ��
| Ԫ�ر�� | Ԫ��������Ϣ |
| A | �䵥�����ܶ���С������ |
| B | �������Ӵ�������λ�ĸ���ɣ������ǿ�����Ҫ�ɷ�֮һ |
| C | ����������B�������Ӿ�����ͬ�ĵ��Ӳ�ṹ����ԭ�ӵ������������Ǵ�����1/4 |
| D | ����������������ﶼ�����ԣ�����Cͬ���� |
| E | ��Cͬ���ڣ���ԭ�Ӱ뾶�ڸ�������С |
| R | �ж��ֻ��ϼۣ����������Ϊ+6�� |
��1��д������Ԫ�ص����ƣ�C ��E ������B�������ӽṹʾ��ͼ�� ��
��2��B��C��D�ļ����Ӱ뾶�ɴ�С��˳���� ���û�ѧʽ��ʾ����EԪ�ص���ۺ�����Ļ�ѧʽ�� ��
��3��ʵ��֤��D�����������������������ԵIJ��������ǣ� ��
��4����һ�������£�RO32����R2���ɷ�����Ӧ��RO32����2R2����6H�� = 3R��3H2O�������й������У���ȷ���� ������ţ���
A��Ԫ��Rλ�����ڱ��е�3 ���ڣ���A��
B��R2������ԭ�ӵ��Ӳ�ṹ��ͬ
C��RO32�������ܷ��������з�Ӧ�ж�ֻ����������
D����Ӧ������1 mol RO32�� ��ת�Ƶĵ��ӵ����ʵ���Ϊ4 mol
��5����д���漰����Ԫ�ؼ��������Ӧ�����ӷ���ʽ��Ҫ��1������֮����û� ��2���ڷ���ʽ����Ϊ���ַǽ���������Dz�ͬ����ġ� �� ��
��1��þ ��
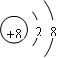 ��2�� O2����Mg2+��Al3+ ��HClO4
��2�� O2����Mg2+��Al3+ ��HClO4��3���������������������зֱ��������NaOH��Һ�����ᣬ���嶼�ܹ��ܽ⡣ ��4��AD ��
��5��Mg��2H��=Mg2����H2������2Al+6H+��2 Al3++3H2������3Mg+2Al3+=3Mg2++2Al��
Cl2��S2��=S����2Cl������Cl2��H2S=S����2Cl����2H����
��������������������֪�⼸��Ԫ�طֱ��ǣ�A��H��B��O��C��Mg��D��Al; E��Cl��R��S����1��CԪ�ص�������þ��EԪ�ص�����Ϊ�ȣ���2��O��Mg��Al�����ӵĵ��Ӳ�ṹ��ͬ������2��8���Ų������ڵ��Ӳ�ṹ��ͬ������˵�����ӵĺ˵����Խ�����ӵİ뾶��ԽС�����Լ����Ӱ뾶�ɴ�С��˳����O2����Mg2+��Al3+��ClԪ�ص�ԭ���������7�����ӣ�����ϼ�Ϊ+7�ۣ���������ۺ�����Ļ�ѧʽ��HClO4����3��֤��Al(OH)3�������ԵIJ�����������Al(OH)3�зֱ��������NaOH��Һ�����ᣬ���嶼�ܹ��ܽ⡣�Ϳ�֤������4��A�� S�ĺ�������Ų���2��8��6�����Ԫ��Rλ�����ڱ��е�3 ���ڣ���A�塣��ȷ��B��S2����Arԭ�ӵ��Ӳ�ṹ��ͬ������C��SO32�����еķ�Ӧ�о��������ԣ����еķ�Ӧ�б��ֻ�ԭ�ԡ�����D������������ԭ��Ӧ�еĵ���ת����Ŀ��ȿ�֪�ڷ�ӦRO32����2R2����6H�� = 3R��3H2O�У�ÿ����1 mol RO32�� ��ת�Ƶĵ��ӵ����ʵ���Ϊ4 mol����ȷ����5�����������������Ӧ�����ӷ���ʽ��Mg��2H��=Mg2����H2����Cl2��S2��=S����2Cl���ȡ�

��ϰ��ϵ�д�
�����Ŀ
